JAVA
Hazard to Self
 
Posts: 71
Registered: 9-1-2014
Member Is Offline
Mood: No Mood
|
|
Best way to make TsCl from TsOH ?
I'am reading so much about how to make "acyl chlorides". In old textbooks you find a huge amount of chlorination techniques including PCl5
(nasty stuff and impossible to buy) POCl3, PCl3 and SOCl2.
Since red P is nearly impossible to get, I focus me on sulfur chlorides. I did read a post about the preparation of Thionyl chloride by using sulfur
trioxide and an excess of SCl2. But what I also did read is that SCl2 is strong enough to break the -OH group and convert it to
TsCl.
I need TsCl as a protection group for aniline to perform a FC acylation. So my creativity don't stop at this point. I have also
P4O10 in the lab and made about 500g TsOH already. My idea is to let TsOH dehydrate to the corresponding tosylate anhydride (see
picture)
Are such a tosylate anhydrides a good alternative for TsCl if you work with pyridine as a solvent to do this job ?
There are much higly experienced chemists here, then my question is: what synthesis is the best one to perform this job ? Someone with experience?
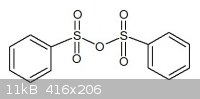
|
|
|
JAVA
Hazard to Self
 
Posts: 71
Registered: 9-1-2014
Member Is Offline
Mood: No Mood
|
|
I'am sorry but after a while it seems that I couldn't edit my message.
I do talk about two ways to achieve my goal:
-> the preparation of Tosyl anhydride with P2O5 but I forgot to draw the two -CH3 groups on it. It's quite easy to
make 'in a pinch' since it have a mp. 125°C, while p-TsOH have a mp. of 68°C.
By now, you see a anhydride with 4 carbonyl groups instead of 2, is that even more reactive ?
-> Wondering that no members are able to convert p-TsOH to p-TsCl with "sulfur chlorides"...
Can't find it back in the recent versions of the CRC, while it is quite important in organic chemistry.
I really appreciate some help.
|
|
|
Brain&Force
Hazard to Lanthanides
    
Posts: 1302
Registered: 13-11-2013
Location: UW-Madison
Member Is Offline
Mood: Incommensurately modulated
|
|
Would this reaction be possible? I'm taking a wild guess:
Ts2O + HCl → 2TsCl + H2O
Obviously the HCl would have to be anhydrous for this to work properly. And the produced water may interfere with the reaction.
At the end of the day, simulating atoms doesn't beat working with the real things...
|
|
|
Aconite
Harmless

Posts: 11
Registered: 23-12-2013
Member Is Offline
Mood: No Mood
|
|
If you can prepare the anhydride easily, that works just as well as (and in some cases better) the acyl chloride for amine protection. I recommend
using that instead of trying to make the chloride.
If you are hell bent on preparing the tosyl chloride, thionyl chloride is typically used to covert acids to acid chlorides.
Tosyl chloride is a byproduct of saccharin synthesis- so if you really want a circuitous route, just make saccharin! Hehehe.
Alternatively, if it isn't any trouble, you can purchase for a decent price benzenesulfonyl chloride (BsCl; besyl chloride) which is actually slightly
more reactive than tosyl chloride and available on electronic Bays, of sorts.
~Cheers
|
|
|
JAVA
Hazard to Self
 
Posts: 71
Registered: 9-1-2014
Member Is Offline
Mood: No Mood
|
|
Thank you for all the clever idea's.
Indeed, tosyl anhydride can not be made by stong dehydratation only by the reaction of tosyl chloride with TsONa. Never seen tosyl anhydride in my
whole life.
I think I succeed in the preparation of TsCl, it melts at 335K which is pretty low compared to TsOH. It's very soluble in DCM, ether, EtOAc but not
very soluble in MeOH or EtOH.
Enjoy your Monday
|
|
|