DubaiAmateurRocketry
National Hazard
   
Posts: 841
Registered: 10-5-2013
Location: LA, CA, USA
Member Is Offline
Mood: In research
|
|
Same name different structure ?
I took a screen shot of the lecture paper and the same product on sigma alreich.
I am confused which one is the correct one ?
<img src="http://i40.tinypic.com/2mm65o7.jpg" width="800" />
<!-- bfesser_edit_tag -->[<a href="u2u.php?action=send&username=bfesser">bfesser</a>: reduced
image size(s)]
[Edited on 4.1.14 by bfesser]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
What exactly is your question?
It is perfectly normal that equal structures bear an equal name. What else did you expect?
|
|
|
DubaiAmateurRocketry
National Hazard
   
Posts: 841
Registered: 10-5-2013
Location: LA, CA, USA
Member Is Offline
Mood: In research
|
|
However the diol groups are placed differently ? I need the compound in the paper for further uses. So how do I know if I shall just buy this or I
shall synthesized it my own ? It has same name, same name however different structure.
|
|
|
Pulverulescent
National Hazard
   
Posts: 793
Registered: 31-1-2008
Member Is Offline
Mood: Torn between two monikers ─ "hissingnoise" and the present incarnation!
|
|
| Quote: | | ]However the diol groups are placed differently ?. |
Two perspectives ─ one molecule . . .
"I know not with what weapons World War III will be fought, but World War IV will be fought with sticks and stones"
A Einstein
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by DubaiAmateurRocketry  | | However the diol groups are placed differently ? I need the compound in the paper for further uses. So how do I know if I shall just buy this or I
shall synthesized it my own ? It has same name, same name however different structure. |
You are not even funny. There is no difference at all. Both structure are completely identical. If you can't read chemical structures, you should not
be messing with chemistry.
|
|
|
ScienceHideout
Hazard to Others
  
Posts: 391
Registered: 12-3-2011
Location: In the Source
Member Is Offline
Mood: High Spin
|
|
DAR- I easily see the source of your confusion. We are always spoiled with those 3d-ish stereo molecules with bonds that jump out at you and stuff,
and as soon as something is smooshed down into 2d it is rather confusing-
Here is how to clear it up... It always works for me 
Build a model of this molecule. I don't care if you use a molecular model set or just a computer simulation like Avogadro. Once you build it, focus on
the middle carbon. You will soon see that there is nothing different between these 2 molecules, it is just how you are looking at it  . .
Hope that helps,
SciHide
hey, if you are reading this, I can't U2U, but you are always welcome to send me an email!
|
|
|
DubaiAmateurRocketry
National Hazard
   
Posts: 841
Registered: 10-5-2013
Location: LA, CA, USA
Member Is Offline
Mood: In research
|
|
Ahh right i understood, I thought it was something else. Thank you so much!
Quote: Originally posted by ScienceHideout  | DAR- I easily see the source of your confusion. We are always spoiled with those 3d-ish stereo molecules with bonds that jump out at you and stuff,
and as soon as something is smooshed down into 2d it is rather confusing-
Here is how to clear it up... It always works for me 
Build a model of this molecule. I don't care if you use a molecular model set or just a computer simulation like Avogadro. Once you build it, focus on
the middle carbon. You will soon see that there is nothing different between these 2 molecules, it is just how you are looking at it  . .
Hope that helps,
SciHide |
Yup I realized, thanks.
and nicodem, sorry for being so stupid, im poor in many parts of chemistry like this one.
[Edited on 4-1-2014 by DubaiAmateurRocketry]
|
|
|
ScienceHideout
Hazard to Others
  
Posts: 391
Registered: 12-3-2011
Location: In the Source
Member Is Offline
Mood: High Spin
|
|
Quote: Originally posted by DubaiAmateurRocketry  |
Ahh right i understood, I thought it was something else. Thank you so much!
Quote: Originally posted by ScienceHideout  | DAR- I easily see the source of your confusion. We are always spoiled with those 3d-ish stereo molecules with bonds that jump out at you and stuff,
and as soon as something is smooshed down into 2d it is rather confusing-
Here is how to clear it up... It always works for me 
Build a model of this molecule. I don't care if you use a molecular model set or just a computer simulation like Avogadro. Once you build it, focus on
the middle carbon. You will soon see that there is nothing different between these 2 molecules, it is just how you are looking at it  . .
Hope that helps,
SciHide |
Yup I realized, thanks.
and nicodem, sorry for being so stupid, im poor in many parts of chemistry like this one.
[Edited on 4-1-2014 by DubaiAmateurRocketry] |
There is a big difference between being unaware and being stupid... You can't fix stupidity! Being unaware is absolutely forgivable in chemistry, and
imagining 3d things on flat pieces of paper is something that comes with much practice and experience. I find it really sad that someone would rather
insult you and make you believe that you are stupid than help you understand such a topic.
I am glad that you understand this now... and btw- don't call yourself stupid  .
Just by studying chem alone you prove you are not stupid .
Just by studying chem alone you prove you are not stupid  ! !
hey, if you are reading this, I can't U2U, but you are always welcome to send me an email!
|
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
Those are both terrible drawings, but they do represent the same molecule. The quaternary carbon (the one with three carbons and a nitrogen bonded to
it) is not a <a href="http://en.wikipedia.org/wiki/Stereocenter" target="_blank">stereocenter</a> <img src="../scipics/_wiki.png"
/>, as two of the attached groups (–CH<sub>2</sub>OH) are identical. It'd be nice to see the molecule represented with solid
and dashed wedges, though, or at the very least with decent bond angles.
[edit] What's worse is that the '3D' models that come up with a <a
href="https://www.google.com/search?q=2-ethyl-2-nitro-1,3-propanediol&safe=off&source=lnms&tbm=isch&sa=X&ei=Iz7IUsSKA6SI2wWHlYCgAw
&ved=0CAkQ_AUoAQ&biw=1317&bih=655" target="_blank">Google search</a> <img src="../scipics/_ext.png" /> represent it as
planar! Not even wrong...
<table><tr><td align="center">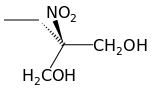 </td></tr><tr><td><em>2-nitro-2-ethylpropane-1,3-diol</em></td></tr></table> </td></tr><tr><td><em>2-nitro-2-ethylpropane-1,3-diol</em></td></tr></table>
I couldn't get the software to cooperate—first time using it—but you get the idea. Or autism. 
[Edited on 4.1.14 by bfesser]
|
|
|
ScienceHideout
Hazard to Others
  
Posts: 391
Registered: 12-3-2011
Location: In the Source
Member Is Offline
Mood: High Spin
|
|
I attached a better picture that I made using marvin... It is a ball and stick model, I would love for someone to post one with wedges, but I can't
figure out how to do that... 
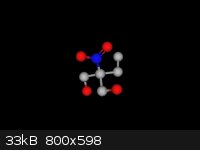
LOL- I just now noticed that this completely lacks all hydrogens 
[Edited on 4-1-2014 by ScienceHideout]
hey, if you are reading this, I can't U2U, but you are always welcome to send me an email!
|
|
|
bfesser
|
Thread Moved
4-1-2014 at 09:32 |