| Pages:
1
2 |
APO
National Hazard
   
Posts: 627
Registered: 28-12-2012
Location: China Lake
Member Is Offline
Mood: Refluxing
|
|
2,5-Dibromo-1,4-benzoquinone Reactivity?
Will 2,5-Dibromo-1,4-benzoquinone react with Sodium Hydroxide or Sodium Methoxide? I can't find any free literature on 2,5-Dibromo-1,4-benzoquinone's
reactivity towards bases at all!
"Damn it George! I told you not to drop me!"
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
It should behave like 2,3,5,6-tetrabromo-1,4-benzoquinone (bromanil).
The chemistry behind must be the same as for chloranil.
The halogen atoms are very easily exchanged by nitrite, hydroxyl or azido anions; one get from this nitranilic acid
(2,5-dihydroxy-3,6-dinitro-benzoquinone), 2,3,5,6-tetrahydroxy-1,4-benzoquinone and 2,3,5,6-tetraazido-1,4-benzoquinone.
If you use the search engine there is a tread of Axt about nitranile and nitranilate salts.
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
I have myself reacted chloranal with sodium methoxide in methanol (refluxed). I will try to recall some details for you from memory (it was many years
ago):
Although the chloranil didn't dissolve well in the concentrated sodium methoxide/methanol solution I was using, it netherless reacted nicely in time
forming a yellow mixture of precipitate and solution under stirring with a bar magnet that also kinda milled the solids on the bottom as it stirred to
form a slurry.
After running for some time (can't remember how long) I filtered and washed with an organic solvent (can remember which but probably methanol) to
[ultimately] recover a red crystalline flake product highly soluble in organic solvents and a bright white amorphous powder after washing heavily at
the filter with water (it was completely insoluble in everything I could try).
IR suggested that the red soluble flakes was indeed tetramethoxyquinone. No surprise, it looked very much like chloranil shiny plates, simply a
beautiful deep red colour.
The question was what was the second super bright white amorphous looking insoluble in everything product  I had made a lot of that too. I had made a lot of that too.
IR of this material suggested it was also heavily methoxylated, but it had clear hydroxyl note on the IR, so I concluded I had over methoxylation here
attacking the carbonyl as well and upon washing it released the sodium and formed a hemiacetal that proved to be very insoluble and very pretty white
and non-crystalline.
This should be reversible then and indeed, heating the white product in a test tube under flame caused vigorous boiling upon melting yielding the same
red crystalline product in time as previously obtained (the tetramethoxyquinone). I am thus fairly sure that methanol was eliminated from the
hemiacetal (seen as a vigorous boiling) and so this was no problem and I could convert the white product into what I wanted easily.
Long story short, don't be surprised if you possibly obtain a hellishly insoluble white product, in fact, it may be worthwhile to use excess methoxide
and target it specifically as it's unavoidable. It is easier to isolate this thing by making use of its extreme insolubility, just wash with copious
water, dry and then do the methanolysis by heating and then recrystallise your quinone product.
Hope my suggestions prove useful.
The structures of the two products that I believe were made are:
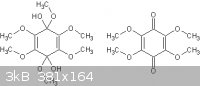
[Edited on 14-11-2013 by deltaH]
|
|
|
Nicodem
|
Thread Moved
14-11-2013 at 06:38 |
APO
National Hazard
   
Posts: 627
Registered: 28-12-2012
Location: China Lake
Member Is Offline
Mood: Refluxing
|
|
Does 2,5-Dibromo-1,4-benzoquinone react with Diethylamine or Diethyl Ether? I know, this is tedious.
"Damn it George! I told you not to drop me!"
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
It would really help if you could give a bit more info APO, what are you after, a base catalyst that won't condense with
bromoquinones as well as a solvent system for it?
If so, may I suggest you read up on non-nucleophilic / sterically hindered organic bases. This will also answer your question about diethylamine.
The solvent suitability depends somewhat on the reaction you are attempting as well as the nature of the reagents/products, so again, info please...
[Edited on 14-11-2013 by deltaH]
|
|
|
APO
National Hazard
   
Posts: 627
Registered: 28-12-2012
Location: China Lake
Member Is Offline
Mood: Refluxing
|
|
I'm sorry, but I can't find anything on Diethylamine's reactivity towards bromoquiones. I'm guesing Diethyl Ether will just act as a solvent for them?
All I know, is that most non-nucleophilic bases decompose 2,5-Dibromo-1,4-benzoquinone on site, such as 1,8-Diazabicyclo[5.4.0]undec-7-ene.
The reason why I want to know is because I came across a reaction that would make a suitable precursor for Cubane 1,3,5-Tricarboxylic Acid, it
involved condensing 2,5-Dibromo-1,4-benzoquinone with Bromocyclopentadienone.
The problem is that the Bromocyclopentadienone must be made in situ while in the presense of the 2,5-Dibromo-1,4-benzquinone so that it will condense
with the 2,5-Dibromo-1,4-benzoquinone, instead of spontaniously dimerizing.
Bromocyclopentadienone is made by base catalysed dehydrohalogenation of Tribromocyclopentanone, but I'm unaware of any reactants that will do this
without reacting with 2,5-Dibromo-1,4-benzoquinone.
So pretty much I need a base catalyst that's inreactive towards bromoquinones, something that will elimanate the two extra bromine atoms on the
Tribromocyclopentanone while leaving the ones on the 2,5-Dibromo-1,4-benzoquinone alone, as well as a solvent for all of this to take place.
"Damn it George! I told you not to drop me!"
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Now it makes more sense, thanks for the info.
Okay, a good base catalyst for this from my experience might be DABCO. It usually plays nicely with quinones. I can't gaurantee it specifically with
your bromoquinone, but I have personally used it as catalyst for a very neat synthesis that made 2,5-dimethoxy-1,4-benzoquinone from paraformaldehyde
in methanol refluxing for several hours with p-benzoquinone and DABCO catalyst (yields and selectivity were okish... but nevertheless).
In regards to solvent, my 2,5-dimethoxy-1,4-benzoquinone was stubbornly insoluble, I don't know if your bromo version will behave in the same way.
Anyhow, the only half decent solvent for it that I found was DCM, however, that was not for a reaction where strong bases were present! This is a big
problem with things like DCM of course.
So as for a solvent, I hope you don't have the same kind of solubility as I had with the dimethoxy derivative, else you've got a problem.
[Edited on 15-11-2013 by deltaH]
|
|
|
APO
National Hazard
   
Posts: 627
Registered: 28-12-2012
Location: China Lake
Member Is Offline
Mood: Refluxing
|
|
Thanks, that seems possible.
But what will eliminate the unwanted bromine atoms on the Tribromocyclopentanone though? Of course while leaving the bromoquinone alone. Will the base
catalyst itself work? That's the way Diethylamine functions for the usual dehydrohalogenation.
"Damn it George! I told you not to drop me!"
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
DABCO should do a marvelous job of elimination the bromines for you, but then you will need it in stoichiometric amounts and not catalytic.
In that case perhaps you'd do much better with triethylamine, it's so much cheaper, especially if you will be needing stoichiometric amounts of base!
[Edited on 15-11-2013 by deltaH]
|
|
|
APO
National Hazard
   
Posts: 627
Registered: 28-12-2012
Location: China Lake
Member Is Offline
Mood: Refluxing
|
|
Wonderful, thanks.
So, just to be clear, DABCO or Triethylamine will work as a base catalyst, remove the unwanted bromines from the Tribromocyclopentanone, all while
leaving the bromoquinone alone?
Also, still need a solvent, preferably not Methylene Chloride.
[Edited on 16-11-2013 by APO]
"Damn it George! I told you not to drop me!"
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
| Quote: | | So, just to be clear, DABCO or Triethylamine will work as a base catalyst, remove the unwanted bromines from the Tribromocyclopentanone, all while
leaving the bromoquinone alone? |
No, these MIGHT work, you won't know till you try  The best is to follow a known
procedure that has been shown to already work if you want to maximise your chances of success. Failing that, DABCO or TEA are probably half reasonable
trial choices but are needed in stoichiometric amounts to affect dehydrohalogenation. The best is to follow a known
procedure that has been shown to already work if you want to maximise your chances of success. Failing that, DABCO or TEA are probably half reasonable
trial choices but are needed in stoichiometric amounts to affect dehydrohalogenation.
The worry with chloromethanes is the possibility of forming reactive carbenes by the special dehydrohalogenation of an alpha proton... HOWEVER, this
is not an easy task at all and requires super bases which DABCO and TEA aren't, so on second thought, DCM might in fact be fine.... again
experimentation will tell all!
As you said, I would prefer you don't use DCM, but if you must for solubility reasons, it may well work just fine. So ether is first choice, failing
that try DCM.
[Edited on 16-11-2013 by deltaH]
|
|
|
APO
National Hazard
   
Posts: 627
Registered: 28-12-2012
Location: China Lake
Member Is Offline
Mood: Refluxing
|
|
Ok, thanks.
Any idea of which may give highest yield?
Regarding DABCO and TEA of course.
[Edited on 16-11-2013 by APO]
"Damn it George! I told you not to drop me!"
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Probably DABCO, but that is speculation! Incidentally, DABCO is one of a few simple amines that has a pleasant odour... smells like peanuts if I
remember correctly.
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
DABCO is very similar in its reactivity to DBU, only weaker, so this might not be a good choice.
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
APO
National Hazard
   
Posts: 627
Registered: 28-12-2012
Location: China Lake
Member Is Offline
Mood: Refluxing
|
|
So what do you think would work vulture?
"Damn it George! I told you not to drop me!"
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
I'm having a hard time following this synthesis, could you draw a scheme?
By which mechanism do bases like DBU attack the benzoquinone?
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
APO
National Hazard
   
Posts: 627
Registered: 28-12-2012
Location: China Lake
Member Is Offline
Mood: Refluxing
|
|
I could draw the theoretical reaction of which would be wanted, and of how it would be approached if that helps.
I don't know how DBU reacts with 2,5-Dibromo-1,4-benzoquinone, but from what I've read DBU tends to decompose it before can undergo the wanted
reaction.
If DeltaH is right that DABCO and TEA typically don't react with bromoquinones, then what would take place is that the DABCO or TEA would react with
Tribromocyclopentanone, which would give Bromocyclopentadienone. However, Bromocyclopentadienone quickly dimerizes on it's own, which is not wanted.
The wanted reaction is for the Bromocyclopentadienone produced to condense with 2,5-Dibromo-1,4-benzoquinone to give an endo adduct that can undergo
photolysis and Favorskii Rearangment to give Cubane 1,3,5-Tricarboxylic Acid. This requires the 2,5-Dibromo-1,4-benzoquinone to be in solution as the
Bromocyclopentadienone is made, so that it undergos the wanted reaction, rather than dimerizing.
So ultimantly, reactants that still undergo dehydrohalogenation with Tribromocyclopentanone, but are inreactive towards bromoquinones are needed.
"Damn it George! I told you not to drop me!"
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Amines tend to readily attach to the ring position of quinones under basic conditions, accompanied by the reduction of the quinone to it's
hydroquinone form. So for DBU, the fast reaction you have read about is probably the formation of:
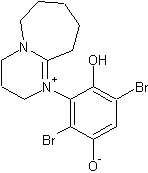
While for some reason the DABCO version is not favoured, probably because of steric hindrance. The DABCO equivalent that would have formed would be:
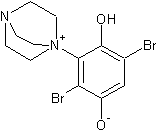
In this animation of the 3d structure, the extreme steric hindrance is clear (click on structure to see animation):
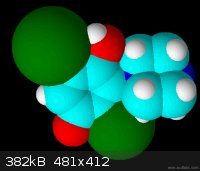
[Edited on 18-11-2013 by deltaH]
|
|
|
APO
National Hazard
   
Posts: 627
Registered: 28-12-2012
Location: China Lake
Member Is Offline
Mood: Refluxing
|
|
Thank you DeltaH, that's very helpful.
Below is a rough draft of the proposed reaction scheme:

The question mark specifies where the suitable reagents from this threads' conclusions will come into play.
"Damn it George! I told you not to drop me!"
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
1) Do we have any references for this attack of amines on quinones? If yes, please supply. The proposed reaction involves a proton transfer which I
would suspect to be hard under aprotic conditions.
2) I don't see how DABCO would cause more steric hindrance than DBU.
3) You obviously got this reaction from the literature, again, a reference would be nice, plus, which conditions do they use?
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Quote: Originally posted by vulture  | 1) Do we have any references for this attack of amines on quinones? If yes, please supply. The proposed reaction involves a proton transfer which I
would suspect to be hard under aprotic conditions.
2) I don't see how DABCO would cause more steric hindrance than DBU.
3) You obviously got this reaction from the literature, again, a reference would be nice, plus, which conditions do they use? |
(1) I worked with quinone addition reaction some several years ago, references are on an old laptop that I have to dig out. It's very old chemistry
(~100 years if I recall from an organic chemistry textbook). I tried a quick google search now, but couldn't find anything, so I will describe what I
can and maybe the op can do better if he is so inclined.
My apologies, but I am very busy this week so can only dedicate more time to this next week. I'll describe the reaction and perhaps either the op or
someone else can chase this up:
The reaction that got me into quinone chemistry was in fact a condensation between aniline and benzoquinone with a base catalyst (this is the 'very
old chemistry' bit). Aniline adds on the 2 position via nitrogen, forming a hydroquinone derivative that then get's reoxidised in situ by
unsubstituted quinone (which is a stronger oxidant) and then the addition happens again on the 5 position, followed by another reoxidation. In the
end, you end up with a 2,5 aniline derivatized quinone and require at least 1.5 equivalents of quinone to aniline and form 1 equivalent underivatized
hydroquinone as a co-product.
I have also done this with melamine as the amine and formed pitch black polymers from this type of reaction. I have filed a patent for that
incidentally, turns out they were excellent support materials for metal carbonyl cluster based catalysts.
Toma, N.V., University of Cape Town (2012), Stabilised and activated metal cluster complex based catalysts and their preparation, Int Pat Appl No
PCT/IB2012/056681.
In fact it's not just amines, quinone chemistry in general tend to form 2,5 addition products in many variations, even the chloranil synthesis from
conc. HCl and H2O2 (patent) proceeds via an isolatable 2,5-dichloroquinone.
As I've said before, I've also prepared a 2,5-dimethoxy-benzoquinone by DABCO catalysed addition in methanol and paraformaldehyde. I did have success
with a google search for you on that:
Colletti, R. F., Stewart, M. J., Taylor, A. E., MacNeill, N. J. and Mathias, L. J. (1991), 2,5,-Dimethoxy- and 2,5-di-n-butoxy-1,4-benzoquinone
reactions and polymerization with 1,6-hexanediamine. J. Polym. Sci. A Polym. Chem., 29: 1633–1638. doi: 10.1002/pola.1991.080291113
I know you are hungry for more, but I am afraid that is the best I can do for now.
(2)So in the case of DBU, since I know that ring additions on the 2 and 5 positions occurs readily with quinones under basic conditions, assuming DBU
reacts readily with quinone as the op suggested, this was merely my suggestion of what might be happening. DBU is bulky, but it can twist 90deg. to
the quinone and so 'slot' in between the bromine and -OH (not easy, but hypothetically possible). Recall the double bond on DBU is resonant between
the two nitrogens making rotation possible.
DABCO, on the other hand, is a bulky 3d 'cage', it cannot twist into any kind of better fit as my animation shows, which is why I would guess it may
be a better choice than DBU.
Finally, in regards to your point about protic versus non-protic solvents, you may be right, however, I would not dismiss this so quickly in aprotic
solvents. In the end of the day, it either happens or it doesn't, for whatever reasons.
[Edited on 18-11-2013 by deltaH]
|
|
|
APO
National Hazard
   
Posts: 627
Registered: 28-12-2012
Location: China Lake
Member Is Offline
Mood: Refluxing
|
|
Thanks for the additional info DeltaH.
Vulture, the literature that suggests the target reaction, and mentions the reactivity of DBU with bromoquinones is here, however, all of there approaches to make the endo adduct failed. They tried a somewhat similar approach, but with much too reactive
reagents.
So, vulture, if aprotic solvents aren't very favorable, what do you suggest?
Also, If you don't think DABCO or TEA will work, do you have an idea of what will?
"Damn it George! I told you not to drop me!"
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
I had a quick look at the file, but not long enough to say anything definitive.
It seems that they completely abandoned the route you are proposing, correct?
What do you mean with "much too reactive reagents"?
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
APO
National Hazard
   
Posts: 627
Registered: 28-12-2012
Location: China Lake
Member Is Offline
Mood: Refluxing
|
|
I wouldn't say they abandonded it, as I don't know if they even thought of it. But yeah, what I'm proposing is considerably different.
What I mean by "much too reactive" is that they tried highly reactive bases, like Potassium Tertiary Butoxide, and pretty much methods that I doubt
whould have a chance of working in the first place.
However, the reaction that shows how to get to the endo adduct, and ultimantly Cubane 1,3,5-Tricarboxylic Acid, was originally thought of by Eaton, so
I think what's presented is still valid.
"Damn it George! I told you not to drop me!"
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Hi APO
Back from my trip and managed to get my old laptop working (it's power supply was another story). Anyhow, the info is from Organic Chemistry by
Finar, I.L (1959), 3rd ed., pp.639-645
I couldn't find this book online, but I found a later edition and browsing through in about the same area, found the same info, you can read this
online at:
https://archive.org/stream/OrganicChemistryVol1/Finar-Organi...
That page specifically discusses this ring addition chemistry of aniline to benzoquinone and from a few pages before gives a nice introductory
discussion about quinone chem in general, you may find it useful in your quinone chemistry.
Hope that helps you!
[Edited on 22-11-2013 by deltaH]
|
|
|
| Pages:
1
2 |