Jmap science
Yet another ID of SimpleChemist-238

Posts: 40
Registered: 19-10-2013
Location: Florida
Member Is Offline
Mood: No Mood
|
|
Oxidation of salicylic acid to 1,2 benzoic acid
Because salicylic acid acts as a alcohol is it possible to use KMnO4 to further oxidize the hydroxyl group to a carboxylic acid, yielding MnO2 and
Potassium 1,2 benzoic acid. Im just a beginner in organic chemistry. 
|
|
|
DraconicAcid
International Hazard
    
Posts: 4334
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Where would the extra carbon come from? Salicylic acid is a phenol- an oxidizing agent such as KMnO4 is not going to do the conversion ROH
-> RCO2H.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Lambda-Eyde
National Hazard
   
Posts: 860
Registered: 20-11-2008
Location: Norway
Member Is Offline
Mood: Cleaved
|
|
No. If you study the structures of the two side by side (and understand what the structural formulae tell you) you should be able to figure out why.
Also, such topics belong in the "Beginnings" section.
This just in: 95,5 % of the world population lives outside the USA
Please drop by our IRC channel: #sciencemadness @ irc.efnet.org
|
|
|
Nicodem
|
Thread Moved
6-11-2013 at 10:47 |
Hexavalent
International Hazard
    
Posts: 1564
Registered: 29-12-2011
Location: Wales, UK
Member Is Offline
Mood: Pericyclic
|
|
I'm feeling quite patient this evening, so I've made a quick reaction scheme for what you are proposing, showing all of the carbon atoms in addition
to the standard functional groups and terminal carbons. As you can see in red and as DraconicAcid
pointed out, a carbon magically appears in your product, which is impossible.
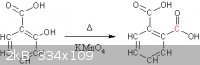
[Edited on 6-11-2013 by Hexavalent]
"Success is going from failure to failure without loss of enthusiasm." Winston Churchill
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
In 2053 the nobel prize for chemistry shall have been awarded (sorry tenses get very complicated with time travel) for the development of a
homogeneous catalyst that inserts CO resulting in the conversion of a phenolic group to an aromatic carboxylic acid.
In his acceptance speech, said winner will have gone on to state that inspiration for this came from a science forum some forty years prior 
EDIT:
Huh... I've just checked the rule book and apparently while I am not permitted to give the details of this, I am permitted of giving abstract
clues about it... so here goes:
[Edited on 7-11-2013 by deltaH]
|
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
Alright, the OP's question has been answered, and this thread is now obsolete. [closed]
<img src="../scipics/_warn.png" /> deltaH, save the spam for Whimsy. <img src="../scipics/_warn.png" />
|
|
|