BobD1001
Hazard to Others
  
Posts: 182
Registered: 29-3-2013
Member Is Offline
Mood: No Mood
|
|
Thermal Decomposition of Urea - Products?
I am interested in determining what Urea thermally decomposes into when heated above its melting point. I just received some Urea and out of boredom
decided to thermally decompose a small amount of it. The smell of Ammonia was quite apparent however I cannot find any sources that state all thermal
decomposition products. I'm also somewhat nervous, seeing that Cyanide's and urea go hand in hand, in the original synthesis or Urea at least. Just
wanted to be sure I wouldn't kick the bucket in a matter of minutes 
|
|
|
Variscite
Hazard to Self
 
Posts: 69
Registered: 21-5-2013
Member Is Offline
Mood: diffusing
|
|
Always check the references before you start messing with chemicals, I would reccomend. Did you heat it on the open air? How hot did you get it?
http://www.sciencedirect.com/science/article/pii/S0040603104...
Something I found with a quick google search.
|
|
|
BobD1001
Hazard to Others
  
Posts: 182
Registered: 29-3-2013
Member Is Offline
Mood: No Mood
|
|
It was heated in open air by heating from the bottom using a propane burner with the sample in an aluminum foil weigh boat. Approx .25g was
decomposed. Its been well over an hour now, and so far no ill effects. Surely not making that kind of negligent mistake again.
|
|
|
Nicodem
|
Thread Moved
24-9-2013 at 07:43 |
Pulverulescent
National Hazard
   
Posts: 793
Registered: 31-1-2008
Member Is Offline
Mood: Torn between two monikers ─ "hissingnoise" and the present incarnation!
|
|
Thermolysis of urea!
"I know not with what weapons World War III will be fought, but World War IV will be fought with sticks and stones"
A Einstein
|
|
|
Boffis
International Hazard
    
Posts: 1867
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
@ Bobd1001
You can prepare Biuret and Cyanuric acid by simply heating urea, the links posted above should give you a good idea of the conditions for simply
heating at different temperatures but gives no work up details. I have made biuret by this technique in the past though I can't lay my hands on my
notes just at the moment but I used a water extraction, urea is more soluble than biuret and cyanuric acid less so. The following preparation is from
a old book on the chemistry of urea that I have:
I. Preparation of Biuret.
The following modification of the procedure described by H. Schiff (1896) gives a very good yield :-
Fifty grams of urea are gently heated in a flat-bottomed flask provided with a cork carrying an inlet and an outlet tube and a thermometer, the bulb
of which is immersed in the molten urea. A current of dry hydrogen chloride is passed over the surface of the urea, while the temperature is gradually
raised to 145°, and maintained at about this point for eighty minutes. The outlet tube is connected to an ordinary tap pump, and a gentle aspiration
is kept up during the heating. The contents of the flask should be given an occasional rotary motion, to prevent caking on the surface. The cold
product is digested, and extracted, with not more than 80 c.c. of water in all, to remove ammonium chloride and unchanged urea. The residue is treated
with 200 c.c. of water and sufficient sodium hydroxide to effect complete solution at a gentle heat. A current of carbon dioxide is passed through the
cold solution, when biuret will be precipitated in the form of thin needle-like crystals. As soon as a granular precipitate appears (sodium dihydrogen
cyanurate), the current of carbon dioxide is stopped. The product is collected and re-crystallised from solution in hot water, or may be purified by
re-solution in sodium hydroxide and precipitation by carbon dioxide. The yield is about 9 grams of the pure substance. More may be recovered from the
first mother liquor after precipitation of the cyanuric acid.
A bit complex but it operates at a lower temperature than without the HCl gas, it also gives you a detailed work-up.
Hope this is helpful. Boff
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Careful decomposition can drive out some ammonia and lead to the formation of biuret, though this is hardly a stoichiometric reaction. Further heating
decomposes urea into the vaporized monomer HOCN. Usually, the monomer quickly condenses to cyanuric acid.
|
|
|
ElectroWin
Hazard to Others
  
Posts: 224
Registered: 5-3-2011
Member Is Offline
Mood: No Mood
|
|
some? yes, about half of the ammonia gets driven off.
|
|
|
testimento
Hazard to Others
  
Posts: 351
Registered: 10-6-2013
Member Is Offline
Mood: No Mood
|
|
I have undestood that urea first decomposes into ammonia and cyanuric acid at 175C. When the cyanuric acid is further heated up to 320-360C, it will
decompose into isocyanic acid, which boils at 23C and in water it will break down into ammonia and carbon dioxide and forming ammonium carbonate in
situ.
|
|
|
Boffis
International Hazard
    
Posts: 1867
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
@testimento, read the links posted above by Pulverescent and Variscite. They are the same source and show that the nature of the product is dependant
on the temperature. Biuret is the first significant product with rising temperature. The idea of the HCl in the experiment I posted is to remove
ammonia from the reaction mixture this assisting the condensation to biuret at a lower temperature and hence reducing the formation of cyanic acid (
and hence cyanuric acid by polymerisation).
|
|
|
testimento
Hazard to Others
  
Posts: 351
Registered: 10-6-2013
Member Is Offline
Mood: No Mood
|
|
Sorry, I forgot to read the few messages in haste.
I quickly drawed this kind of apparatus which could be used to manufacture any of the urea compounds from biuret to isocyanic acid and ammonia. Idea
is to heat the bath up to suitable temperature in order to heat up the urea being dropped into it as fast as possible into the proper temperature to
obtain the desired compound. In my case it is cyanuric acid, so the bath would be heated to 150-175C. The ammonia would be let in at small portions at
a time, from few grams up to few dozens of grams to heat it up as fast as possible. It could be in granulate form or in powder, first being more
suitable for valve. When the process is complete, the bath would be removed and the cyanuric acid heated with full power to decompose it into
isocyanic acid. Essentially the setup is similar to the diethyl ether production via sulfuric acid reduction of ethanol, but with solid reagents.
If the construction was to be utilized in larger production scheme, the ball valve could be replaced with a peppermill-type rotating feeder.
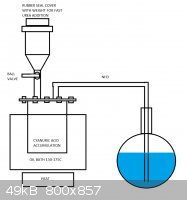
[Edited on 25-9-2013 by testimento]
[Edited on 26-9-2013 by testimento]
|
|
|