| Pages:
1
2
3
4 |
Cyrus
Hazard to Others
  
Posts: 397
Registered: 24-4-2004
Location: Ancient Persia
Member Is Offline
Mood: No Mood
|
|
Crucibles and Metalcasting
Sand casting molten metal is a great way to make stuff, including scientific equipment, think pure silver hydrazine reaction vessel, pure Al tubes,
homemade everything...
However, I need a crucible.
I am trying to make a crucible, and don't want to use steel because the iron will oxidize away until it fails. When crucibles fail, bad things
happen.
I need some mad scientist ways to make a crucible!
Don't tell me use iron (fails) or just fireclay (too weak) or porcelain (too easy ) or a teacup ) or a teacup (too small). I will test these out, and report on my
results though... (too small). I will test these out, and report on my
results though...
The crucible must be about 0.5 L, and it must withstand thermal shock, molten metals, and somewhere around 1000 deg. C.
Many crucibles are fireclay/graphite, SiC, MgO, Al2O3, silicates, or a mixture of the above. How can these be made in the lab?
I have several ideas-
1 a compound that decomposes at high temp to form a crucible material ie MgCO3 ought to eventually turn into MgO. The problem I see is that the MgO
would probably be a powder, not a solid shape.
2 a mixture of chemicals that can be ignited/reacted in a form/etc..., and the residue would be a crucible. I have the crazy idea of taking sugar,
filling a mold with it, sealing the mold except for a few small holes, heating, and taking out a perfectly formed pure carbon crucible... not likely though not likely though 
[Edited on 8-8-2004 by Cyrus]
|
|
|
IvX
Hazard to Others
  
Posts: 112
Registered: 14-4-2004
Member Is Offline
Mood: No Mood
|
|
Why not steel?It would work fine for one time and doesnt cost very much.
Your sugar idea is good and all except the sugar would just melt and fall through the holes.Dip it in sulphuric acid maybe.
I'm watching animal face of now and here top make the (alluminium)skeleton they make it first in styrofoam then put it in a sand box.The sand box
completely covers the styrofoam model except for a small hole with a pipe in it.They then pour the molten AL in(using a steel label btw).It
does'nt give a very precise output though but you might want to try it anyway 
|
|
|
Cyrus
Hazard to Others
  
Posts: 397
Registered: 24-4-2004
Location: Ancient Persia
Member Is Offline
Mood: No Mood
|
|
I have tried steel- it oxidizes away to nothing. That is why I don't want to use it. In a well tuned propane furnace this oxidizing is pretty
slow, but in a charcoal furnace, steel is lunch! (in mine at least)
However, I am in the process of making another steel crucible from a 1 lb
propane tank, if anyone else does this, beware! It looks like the top half and the bottom half are just brazed together in the middle! If you cut
the very top off, and use the rest, there is a high probability that the bottom may drop out! from a 1 lb
propane tank, if anyone else does this, beware! It looks like the top half and the bottom half are just brazed together in the middle! If you cut
the very top off, and use the rest, there is a high probability that the bottom may drop out! To prevent oxidation of the steel, I want to coat it with a protective layer, the best options I can think of are fireplace cement and
fireclay. Any ideas? To prevent oxidation of the steel, I want to coat it with a protective layer, the best options I can think of are fireplace cement and
fireclay. Any ideas?
More details to follow....
Edit, the sugar idea was kind of a joke , I have tried it, you just get hardened
bubbles of carbon usually, but if the holes were at the top, it couldn't leak through them. Another variation would be to make a crucible, soak
it in boiling sugar soln. for a while, and then heat that up. , I have tried it, you just get hardened
bubbles of carbon usually, but if the holes were at the top, it couldn't leak through them. Another variation would be to make a crucible, soak
it in boiling sugar soln. for a while, and then heat that up.
The sulfuric acid is supposed to make a solid chunk of graphite? I have tried it
before and it only makes a loosely held mush of amorphous carbon. 
[Edited on 8-8-2004 by Cyrus]
|
|
|
Democritus of Abdera
Harmless

Posts: 47
Registered: 5-8-2004
Location: The Prefecture of Abdera in the region of Xanthi.
Member Is Offline
Mood: Stone Dead
|
|
Crucible making guide
http://www.lindsaybks.com/dgjp/djgbk/cruc/index.html
Do not meddle in the affairs of wizards, for you are crunchy and taste good with ketchup.\"--- Marcel Marceau
|
|
|
Saerynide
National Hazard
   
Posts: 954
Registered: 17-11-2003
Location: The Void
Member Is Offline
Mood: Ionic
|
|
Cyrus: H2SO4 and sugar doesnt' make a solid block of carbon. It makes this giant bubbly mass that looks extremely disgusting.
"Microsoft reserves the right at all times to monitor communications on the Service and disclose any information Microsoft deems necessary to...
satisfy any applicable law, regulation or legal process"
|
|
|
Cyrus
Hazard to Others
  
Posts: 397
Registered: 24-4-2004
Location: Ancient Persia
Member Is Offline
Mood: No Mood
|
|
I know it turns into mush. I have done it. That's why I was questioning Ivx's idea.
However, flinn scientific (which has been wrong before) says about this reaction, "Your students will be amazed as they watch a yellow
solid-liquid mixture turn brown, then black, expand out the top of the beaker- and solidify. "
I wasn't amazed when I did this reaction, and my product didn't "solidify"! Saerynide, if there was some way to reduce the size
or amount of bubbles, might the carbon turn into a solid mass? Is the carbon produced amorphous or graphite? Maybe some other reaction could be used
to produce the solid graphite, such as heating pitch. I read of a method using pitch or tar to make a graphite foam block!
Democritus, umm, nice link, but I'm not about to buy a book for this, and he suggests using clay, which I am already trying. If you have this
book and know the type/composition of clay and firing conditions he uses, that would be very useful! Thanks.
|
|
|
Saerynide
National Hazard
   
Posts: 954
Registered: 17-11-2003
Location: The Void
Member Is Offline
Mood: Ionic
|
|
When I meant its "not solid" I meant that it was a block which was full of holes, like very holey styrofoam. I wasn't meaning
"mushy slush". I thought you were asking for a rock-solid-hole-free block of graphite. Sorry for the confusion.
To make a hard blob, you need more H2SO4 than sugar (someone posted a good amount a while ago), or else the H2SO4 would hydrate too quickly.
But I dont think this would make a very suitable crucible though. Its a bit too much on the porous side.
"Microsoft reserves the right at all times to monitor communications on the Service and disclose any information Microsoft deems necessary to...
satisfy any applicable law, regulation or legal process"
|
|
|
Democritus of Abdera
Harmless

Posts: 47
Registered: 5-8-2004
Location: The Prefecture of Abdera in the region of Xanthi.
Member Is Offline
Mood: Stone Dead
|
|
Cm'on Cyrus!
If you think that you're going to mix a little acid and sugar together on a substrate and get a graphite crucible, think again. It's not
going to happen.
A graphite crucible about the size of a mans fist sells for about a hundred bucks, the reason for that is that graphite is a relatively rare material
and difficult to work with, a fact hidden by the preponderance of "lead" pencils. Because of the high price of graphite items proffessional
research has been going on for several lifetimes, with an expenditure that must reach figures in the hundreds of millions of dollars in today's
money.
If you really want to go into it, feel free, but I can imagine that most ideas that you could imagine have been thought of and most techniques that
you could try at home have been tried.
Graphite occurs as dispersed geologic deposits(bedded shale or small flakes dispersed in bulky ores) and the processes required to produce graphite
from soft carbon in the laboratory are just slightly less arduous than those required to produce diamonds.
Pencils have become cheap because instead of pressing the graphite powder together under monstrous hydraulic presses to form, they simply mix it with
glues and lightly press it together.
large graphite objects must be pressed with tremendous force and a "large" graphite object is still pretty small if you need the graphite to
be of even consistency throughout.
Crucibles are extremely pricey, turning up your nose at a 10 dollar instruction manual on how to churn them out yourself by the dozens yourself is
silly, because the technology has evolved over the last millenium or so and for you to stumble across the best and cheapest way to make good crucibles
throught trial and error seems to be........ foolish at best.
And someone already took the weeks or months required to gather the info and perfect a process suitable for the DIY'er.
Perhaps if you wish to perform experiments, you might find it profitible to know what is currently already known and being done today and start there,
instead of from scratch.
There is little to be said for a man who spends his time re-inventing the wheel.
It profits neither him personally, nor humanity as a whole.
Besides, wasn't there something you wanted the crucible for? Wouldn't your time be better spent doing whatever that is?
Do not meddle in the affairs of wizards, for you are crunchy and taste good with ketchup.\"--- Marcel Marceau
|
|
|
BromicAcid
International Hazard
    
Posts: 3247
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
In my general chemistry class that I took when I started college we made alumina crucibles. We took aluminum oxide, a tiny amount of water and sodium
silicate and mixed to a paste then pressed it into a form in the shape of a crucible imbedded in a larger brick of what I thought was plaster.
After a few days we broke them out of the plaster and dehydrated them in an oven, when it was all over we had a tiny alumina crucible.
Or take a vessel made of aluminum in the shape of the crucible you want and put it into the bottom of a larger container that is sealed air tight,
pass nickel carbonyl fumes into it (denser then air so they'll sink into the vessel) and make sure the whole thing is heated from the bottom, ta
dah, dissolve the aluminum when you're done and a nickel crucible you have [only 15% serious  ] ]
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
That's interesting, your description of alumina crucibles. Do you have a more detailed description? I.e. proportions of oxide, silicate and h2O?
How resistent is it to cracks (thermal stress), and temperature?
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
BromicAcid
International Hazard
    
Posts: 3247
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Well it was over three years ago but I remember the paste was relatively thick and that everyone who made their crucible walls thick didn't get
them back after they were fired. I have no clue on the proportions.
They were for all intents honest to goodness alumina crucibles, good for many high heat applictions, the ones that actually made it held up nicely for
the advanced chem students I hear and the one that I got when I was in advanced chem served me well.
|
|
|
Twospoons
International Hazard
    
Posts: 1324
Registered: 26-7-2004
Location: Middle Earth
Member Is Offline
Mood: A trace of hope...
|
|
What you describe is similar in many respects to glaze formulations. Essentially you combine a refractory (alumina, silica, zirconia) with a flux (
generally an alkali oxide), the proportions determining the sintering and melting points. The sodium silicate would provide both silica and sodium
oxide to your formula, as well as acting as a plasticiser for the wet mix.
Must dig out my ceramic materials books and see if I can find something more useful 
|
|
|
Cyrus
Hazard to Others
  
Posts: 397
Registered: 24-4-2004
Location: Ancient Persia
Member Is Offline
Mood: No Mood
|
|
THANK YOU BROMIC ACID!!!
BEAUTIFUL! EXACTLY WHAT I WAS LOOKING FOR! IT WILL BE TRIED!
I only have sodium metasilicate though, so the results may be different.
The Nickel Carbonyl idea just scares me...
-start rant-
Democritus, did you write or market that booklet? There is no need to defend it
so much! Why pay 10 bucks to get a booklet if with a little trial and error (which I and most people on this forum enjoy) I can get the same results,
have much more fun, learn lots about how different crucibles are made, gain pottery skill There is no need to defend it
so much! Why pay 10 bucks to get a booklet if with a little trial and error (which I and most people on this forum enjoy) I can get the same results,
have much more fun, learn lots about how different crucibles are made, gain pottery skill , do it my own way, and save ten dollars , do it my own way, and save ten dollars ??!. ??!.
About the acid and sugar, as I have said before, it is a joke! I repeat-
IT IS A JOKE!
I was only about 30 percent serious! Are you going to try to flame BromicAcid for suggesting a farfetched Ni crucible method? If I may quote you,
"C'mon"
After mentioning it, I just wanted to inquire more about the reaction, and see if it just MIGHT be possible. I take it that this inquiry deserves
criticism from you for some reason.
"There is little to be said for a man who spends his time re-inventing the wheel.
It profits neither him personally, nor humanity as a whole. "
Hmm, that includes everyone in this forum who has synthesized a chemical that they could buy easily. This is not the site for you. Sorry. We spend
hours on end doing ancient reactions when we could just buy the products. Why? IT'S FUN!! I'm not making a crucible to benefit humanity,
I'm doing it because I want to!
If you are out to benefit humanity (which is not a bad thing IMO), unplug your computer and do something else. This isn't the site!
"Besides, wasn't there something you wanted the crucible for? Wouldn't your time be better spent doing whatever that is?"
Why don't I just buy a crucible then. In fact why don't I just buy whatever I was going to make (let's say a lathe), and why
don't (to save time and benefit humanity) I just buy whatever I was going too make with the lathe (lets say a rocket nozzle), Hey! WHY DON'T
I JUST BUY AN ESTES ROCKET???
The fun is in the journey, not in the destination!
-end rant-
This may be spoonfeeding , but twospoons, what happens when alumina, and sodium
oxide are heated together, what I mean is what is binding the crucible together? Is the Al2O3 just there for bulk and heat resistance , and the
particles are bound together by a sodium oxide, or sodium silicate glue? Since the melting point of sodium silicate is 1132 deg. C (from google) ,
what happens above these temperatures? I guess the crucible would soften. I am having a hard time finding out what reactions actually happen when
things are fired. , but twospoons, what happens when alumina, and sodium
oxide are heated together, what I mean is what is binding the crucible together? Is the Al2O3 just there for bulk and heat resistance , and the
particles are bound together by a sodium oxide, or sodium silicate glue? Since the melting point of sodium silicate is 1132 deg. C (from google) ,
what happens above these temperatures? I guess the crucible would soften. I am having a hard time finding out what reactions actually happen when
things are fired.
|
|
|
Democritus of Abdera
Harmless

Posts: 47
Registered: 5-8-2004
Location: The Prefecture of Abdera in the region of Xanthi.
Member Is Offline
Mood: Stone Dead
|
|
| Quote: | Originally posted by Cyrus
......The fun is in the journey, not in the destination! ........ a little trial and error..... (which I and most people on this forum enjoy) ......
I can get the same results, have much more fun, learn lots....... ..gain skill...... do it my own way......
twospoons, what happens when alumina, and sodium oxide are heated together? |
TSK! TSK! TSK! .... flip-flopping like John Kerry naked on a greased trampoline! 
Do not meddle in the affairs of wizards, for you are crunchy and taste good with ketchup.\"--- Marcel Marceau
|
|
|
BromicAcid
International Hazard
    
Posts: 3247
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
| Quote: | | I was only about 30 percent serious! Are you going to try to flameBromicAcid for suggesting a farfetched Ni crucible method? If I mayquote
you,"C'mon" |
Actually, the idea was mostly Theoretic's. 
|
|
|
Twospoons
International Hazard
    
Posts: 1324
Registered: 26-7-2004
Location: Middle Earth
Member Is Offline
Mood: A trace of hope...
|
|
I'm not too hot on the actual chemistry of these sintering type things, but I believe the soda acts as a flux, so the melting point of the
mixture ends up lower than its individual components - like tin + lead makes solder with a melt point lower than both.
Also found one reference to graphite crucibles suggesting a 50/50 mix of graphite and kaolin, mixed wet and rammed into a mold. Same site said
"No experimental results yet" so I guess its up to you to test it!
|
|
|
Cyrus
Hazard to Others
  
Posts: 397
Registered: 24-4-2004
Location: Ancient Persia
Member Is Offline
Mood: No Mood
|
|
Ok, several things...
Democritus, lets stop the arguing now, if you want I can continue to wrangle with you till judgement day by u2u, but I doubt it will help anyone. I
don't want to clutter up this board any more with my and your off topic arguments. Thanks.
Back on topic
I "found out" what happens when clays are fired (according to a ceramics guy who I talked to for a while earlier today). The answer,
drumroll please, is LOST WATER! First, the water that was just hangin' around (think wet clay) evaporates, and then the water which was bonded
chemically to any of the molecules in the clay leaves. (As heat is increased the clay goes through a cristoballite sp? stage at about 1200 deg. F,
and then at about 2300 deg. F, the clay is "fully fired" (what this means he did not explain) and then as the clay cools down, it goes
through those same stages again, except the water does not come back!
Huh? Fired clay is the same as unfired clay except it doesn't have any water?
I was asking the guy a lot of questions, so I didn't want to press the issue too much, that is all of the information I got, correct or not.
Anyways, instead of porcelain, which I wanted to get for the crucible, he recommended raku clay- the stuff that is dipped in water while still very
very hot, it ought not to crack from thermal shock if it can take that! If anyone is interested the type I got was Coleman Raku clay. Supposedly,
porcelain (which I think is a synonym for pure kaolin) is so pure that it doesn't resist thermal shock as well as clays that have fireclay added.
Fireclay is a type of clay that is added to other clays to increase thermal shock resistance, but is not good on its own, and the type I got already
had some mixed in.
I also got some alumina for the alumina crucible, (it was labeled alumina oxide, is this just another name for alumina, a mistake, or a different
compound? )
some bentonite for greensand, and potassium dichromate- the guy didn't know what it was for, so I got it for free!
I love pottery stores- have I mentioned that before? Yes I have. 
Next I am going to do some tests!
|
|
|
Twospoons
International Hazard
    
Posts: 1324
Registered: 26-7-2004
Location: Middle Earth
Member Is Offline
Mood: A trace of hope...
|
|
Today I have my book with me "Pottery Materials" by John Colbeck, ISBN 0 7134 46951.
With regards to firing, most clays become irreversibly hardened around 650 C (red heat), as the chemically bonded water is lost. From yellow through
white heat the fluxes in the clay become active, melting and bonding the individual clay particles, and reducing the porosity of the clay body. Once
the clay is completely non-porous it is said to be vitrified.
For your crucible you'll want a high firing clay, generally refered to as 'stoneware'. These clays achieve vitrification in the 1200C
- 1300 C range.
Improved thermal shock resistance can be achieved by adding grog (ground up pre-fired fireclay) to the raw clay. This will also reduce shrinkage of
the clay as it dries, and improve the strength at high temperatures. Grog is typically added in the 10%-30% range (by volume), though you can add
more if you wish.
'Raku clay' is just that - stoneware with lots of grog added. Though the raku firing I've done did not involve tossing hot pots into
water - we used sawdust. Be warned - raku firing does not produce vitrified clay, the firing cycle is too short.
To get a nice stong crucible I suggest you find a friendly potter to fire the thing properly, or join a pottery class. There are two temperatures at
which a decent soak period, and good temperature control are needed - around 100C where the free water is driven off, and around 900C where
carbonaceous material is burned out. For this you really need a properly controlled kiln.
Alumina and aluminium oxide are the same thing
I love pottery stores too - so many fabulous chemicals...
|
|
|
Cyrus
Hazard to Others
  
Posts: 397
Registered: 24-4-2004
Location: Ancient Persia
Member Is Offline
Mood: No Mood
|
|
Hmm, charcoal doesn't really have a temperature gauge. 
I was planning to wait till the clay was dry, and then fire it as hot as I possible could for about 2 hours, this might be a bad idea, it sounds like
the clay has to be heated up to 100 deg, held there for a while, then to 900 deg, held there for a while, and then to full firing temperature.
So the clay will be ruined if I just roast the poor thing after maybe drying it in the oven for a while? Too bad. I'm still going to try it
though, I have plenty of clay.
Tomorrow I am going to attempt to make a crucible on a potter's wheel, today make some plaster crucible molds. (I made the plaster myself btw,
guess how ) )
I had another crazy idea for a crucible, this one is about 64.3% serious.
Take an aluminum casting in the shape of the crucible desired, and anodize the heck out of it. Tada, now it's a pure Al2O3 crucible!
The problems I forsee are that after a while the anodizing might slow down because the oxide layer is so thick, and after a while, the Al layer will
become so thin that some parts may become disconnected from the flow of electricity.
And it might take a while
Here's a pic. to illustrate.
|
|
|
IvX
Hazard to Others
  
Posts: 112
Registered: 14-4-2004
Member Is Offline
Mood: No Mood
|
|
Theres no pic dude.
About your AL2O3 idea you could try burning thermite in something like a crucible.Sand shaped roughly for instance.The metal should form a layer and
the AL2O3 on top of it.
|
|
|
Democritus of Abdera
Harmless

Posts: 47
Registered: 5-8-2004
Location: The Prefecture of Abdera in the region of Xanthi.
Member Is Offline
Mood: Stone Dead
|
|
well...uh...actually...
| Quote: | Originally posted by Cyrus
Hmm, charcoal doesn't really have a temperature gauge. 
|
actually charcoal fired kilns and ovens do, and they are cheap like dirt (but only single use) They are called cones, but are actually tiny pyramids
and you can formulate your own or buy them ready made.
learn more here....  http://www.miniworlddolls.com/evenheat/ConeInfo.htm http://www.miniworlddolls.com/evenheat/ConeInfo.htm
Do not meddle in the affairs of wizards, for you are crunchy and taste good with ketchup.\"--- Marcel Marceau
|
|
|
Democritus of Abdera
Harmless

Posts: 47
Registered: 5-8-2004
Location: The Prefecture of Abdera in the region of Xanthi.
Member Is Offline
Mood: Stone Dead
|
|
| Quote: | Originally posted by Cyrus
I had another crazy idea for a crucible, this one is about 64.3% serious.
Take an aluminum casting in the shape of the crucible desired, and anodize the heck out of it. Tada, now it's a pure Al2O3 crucible!
The problems I forsee are that after a while the anodizing might slow down because the oxide layer is so thick, and after a while, the Al layer will
become so thin that some parts may become disconnected from the flow of electricity.
And it might take a while |
This idea might present a problem, in that you might require a Dr. Frankenstein-like battery of transformer to get the required voltage to pass into
your crucible through the Aluminum Oxide layer, because if I read the attatched table correctly, alumina is quite resistant to the flow of
electrons.
(I had previously researched the techniques and materials for anodizing aluminum and noticed that while thinner coating could be done at home even
slightly thicker ones needed extremely higher soak times and power requirements)
http://www.mkt-intl.com/ceramics/alumina.html
Do not meddle in the affairs of wizards, for you are crunchy and taste good with ketchup.\"--- Marcel Marceau
|
|
|
Cyrus
Hazard to Others
  
Posts: 397
Registered: 24-4-2004
Location: Ancient Persia
Member Is Offline
Mood: No Mood
|
|
Thanks, Democritus. Interesting...
Here is the pic, it didn't load last time and I was much too busy to fix it...
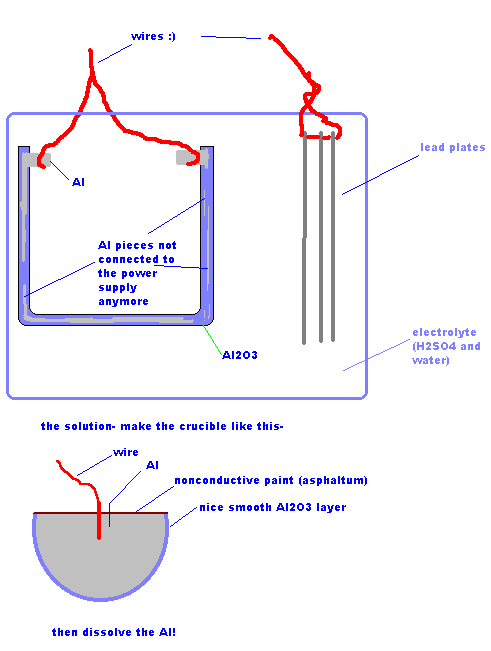
|
|
|
Democritus of Abdera
Harmless

Posts: 47
Registered: 5-8-2004
Location: The Prefecture of Abdera in the region of Xanthi.
Member Is Offline
Mood: Stone Dead
|
|
Hmm.
I took a look at that picture and while I think it might be practically unfeasible, I do have one adjustment to offer to the thought experiment.
I feel it's greatest value lies in the exercise of the mind all concepts give rather than the invention of a new process.
anyway;
In the 2nd picture you posted, it illustrates anodising the bottom of a half-sphere and dissolving the aluminum out, to leave an alumina shell. This
would require a fantastic amount of chemicals and time.
I would suggest foregoing the chemical process at that point and turning to the thermal, the melting point of aluminum oxide is a robust 2072°C
while the melting temperature of aluminum metal is a mere 660.32°C (give or take  ) )
prolonged heating the half-sphere with a propane flame would allow the aluminum metal to be poured out of the half-shell like an egg slipping out of a
shell. 
However; I do not believe this to be readily practicable. The forming of the aluminum oxide half-sphere depends on an aluminum oxide crystal Al2 O3 , occupying the same space and configuration as the closest packed aluminum
metal structure.
And that would defy the accepted "laws" of physics.
Since alumina occupies a much greater space than aluminum metal, I postulate that anything beyond a layer measured in microns would begin to deform
and lose cohesion and structural integrity.
Do not meddle in the affairs of wizards, for you are crunchy and taste good with ketchup.\"--- Marcel Marceau
|
|
|
Cyrus
Hazard to Others
  
Posts: 397
Registered: 24-4-2004
Location: Ancient Persia
Member Is Offline
Mood: No Mood
|
|
Aw, those stupid laws. 
You are probably right, but there is one way to find out, which is currently beyond my means. 
Anyways, melting the metal ought sounds very problematic - when it expands, wouldn't it crack the Al2O3 outer layer instead of smoothly popping
out?
|
|
|
| Pages:
1
2
3
4 |