| Pages:
1
2 |
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Synthesis of Ethyl ß-Naphtholate (Nerolin)
A. Introduction
This procedure is for the preparation of nerolin, a perfume, and scent fixative. It is an alkyl aryl ether and is prepared here using the Williamson
ether synthesis.
ß-naphthol, a weak acid, reacts with potassium hydroxide to form potassium ß-naphtholate:
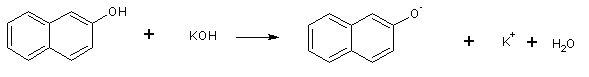
The ß-naphtholate anion then reacts with ethyl iodide by an SN2 mechanism to give ethyl ß-naphtholate:
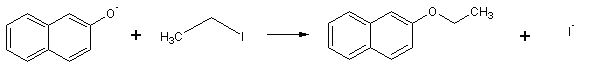
B. Reagents
8g of potassium hydroxide (corrosive!)
6mL of ethyl iodide
10g of ß-naphthol
100mL of methanol (anhydrous)
CAUTION: methanol is highly flammable, and poisonous

reagents
C. Equipment
250mL RBF
condenser (cold water cooled)
heating mantle
magnetic stirrer (optional)
600mL beaker
7cm Buchner funnel
suction flask
vacuum source
25mL Erlenmeyer flask
D. Procedure
Set up the 250mL RBF and condenser for reflux as shown in the photo below. The use of magnetic stirring is optional. However, it assists in mixing
the reactants prior to boiling, and mitigates the mild bumping that occurs in the latter part of the reflux period.

2 hour reflux
Place the methanol, ß-naphthol, KOH, and ethyl iodide in the 250mL RBF. Heat to boiling and reflux for 1.5 to 2 hours.
E. Workup
Fill the 600mL beaker to the 300mL mark with cracked ice. Then pour the hot reaction product onto the ice and stir well. The nerolin will
precipitate as shown in the photo below:

precipitated nerolin on ice
When the ice has melted collect the nerolin by vacuum filtration using the Buchner funnel. Crude nerolin (~5g) is shown in the photo below:

crude nerolin
Recrystallize the nerolin from methanol. You should get some glistening pure white plates as shown in the photo below:

recrystallized nerolin
F. Results
The recrystallized nerolin shown above had a melting point of 36°C. The literature value is 37-38 °C. My yield was only 0.32g.
G. Discussion
Nerolin is used in perfumery and as a fixative in soaps. Fixatives reduce the evaporation rate of other added volatile scents.
One source of the discoloration in the crude nerolin was likely the homemade ß-naphthol, as it is also off-color. During the recrystallization there
was a large amount of contamination that precipitated out first. I carefully removed the upper crystals which looked to be quite pure as shown in the
photo above. I then attempted to harvest more pure crystals from the heavy residue but was not successful.
I’m not sure why the yield of nerolin is so low. Perhaps steric hindrance plays a part as the ß-naphtholate anion is quite bulky. I should also
mention that I accidentally used 150mL of the solvent methanol instead of 100mL, which would reduce the concentration of the reactants.
This is a simple procedure and you are rewarded with a most delightful smelling compound. It smells so good you will not want to wash it off your
hands. I detect notes of vanilla and citrus.
H. Reference
This procedure is a 2X scale paraphrase of a college hand-out obtained in 2003.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Hexavalent
International Hazard
    
Posts: 1564
Registered: 29-12-2011
Location: Wales, UK
Member Is Offline
Mood: Pericyclic
|
|
Excellent work and write-up Magpie, as usual.
Do you have any future plans for your nerolin? Perhaps cleavage with a hydrohalic acid to give an alkyl halide and an alcohol? Or even chlorination to
give the alpha-chloroether? Good luck with your future work, I look forward to reading about more of your experiments.
"Success is going from failure to failure without loss of enthusiasm." Winston Churchill
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Thanks, Hexavalent. No, I have no further plans for the nerolin.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
I wish there was a like function like facebook has just to show interest in threads where my post really wouldn't be of any real value. Either way
always a pleasure Magpie.
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Thank you Sedit - very kind of you.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
reckless explosive
Harmless

Posts: 28
Registered: 1-12-2011
Member Is Offline
Mood: experimental
|
|
Could ethyl bromide be used in place of the ethyl iodide as long as its in the same stoichiometric amounts? As i cannot obtain the chemicals to make
ethyl iodide or buy but i could make ethyl bromide with a raid of thr local pool store.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
See the 2 posts of Nicodem in this thread:
http://www.sciencemadness.org/talk/viewthread.php?tid=11663&...
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
sbbspartan
Hazard to Self
 
Posts: 61
Registered: 6-3-2012
Location: Minnesota, USA
Member Is Offline
Mood: DEAD (diethyl azodicarboxylate)
|
|
For people interested in how to synthesize ß-naphthol, there is some information that might prove useful in this thread:
http://www.sciencemadness.org/talk/viewthread.php?tid=11663
Is that how you made your ß-naphthol Magpie?
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Yes, I describe my preparation down farther in that same thread.
Maybe some don't know that 2-naphthol = beta-naphthol. That's IUPAC nomenclature vs the common name, I believe.
[Edited on 14-2-2013 by Magpie]
[Edited on 14-2-2013 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
sbbspartan
Hazard to Self
 
Posts: 61
Registered: 6-3-2012
Location: Minnesota, USA
Member Is Offline
Mood: DEAD (diethyl azodicarboxylate)
|
|
Funny, I didn't even realize the thread you listed was the same one I was looking at. 
You use the same source of methanol as I do too. I like HEET, it's easy to get and cheap...
Good prep!
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
I too, recently finished the preparation of 2-ethoxynaphthalene via a slightly alternate procedure (that I more or less made up entirely as
modifications of this prep). Instead of KOH, I elected to use sodium methoxide produced freshly as base. My starting 2-naphthol was somewhat less pure
than Magpie's, melting from 118-120C (uncorr, lit. value 123C). This is pictured below.

A 250ml RBF was charged with a stirbar and 75ml of methanol (HEET, distilled to remove the fraction of a percent of oily high-boiling material). 1.33g
(0.058mol) of fresh sodium, cut into thin sticks to speed reaction was added and stirring started. The methanol became quite warm as the sodium
rapidly dissolved to an ever-so-slightly hazy solution of NaOMe in methanol.

7.21g (0.050mol) of the aforementioned (and pictured) mostly-pure 2-naphthol was added to this methoxide solution, rapidly dissolving to a dark brown
solution. A liebig condenser was placed atop the RBF as a reflux condenser. After stirring for ~5 minutes, 10.92g (0.10mol) of Ethyl
Bromide (I don't reccomend this. Just get some iodide. The reflux period is way too long with bromide unless you're making mol
quantities.) was poured down the condenser into the reaction mixture, and the condenser was topped with a CaCl2 drying tube.
Very gentle reflux was maintained for 26 hours (arbitrary, based on the advice of Nicodem in the 2-naphthol thread, I did not have TLC plates to
monitor with) The reaction mixture remained at ~50C during the entire period and at no time was the condenser permitted to rise above 20C through
addition of ice to the recirculating water bath. The color of the reaction mixture lightened over the reflux period, but remained brown.

The reaction mixture was then brought to a vigorous boil for 5 minutes with the condenser removed in order to expel excess EtBr and any ethereal
byproducts. The hot reaction mixture was poured into 300ml of ice water containing 2g of NaOH to help dissolve any unreacted naphthol. Initially, the
mixture was a milky off-white suspension, but with stirring the 2-naphthol slowly "curdled" and sank to the bottom of the beaker leaving a cloudy, but
decidedly translucent supernatant.

This solid was vacuum filtered and washed with 100ml of distilled water. It is highly hydrophobic. Air is drawn through the solid for some time and it
dries fairly easily, providing 7.41g of crude 2-ethoxynaphthalene as a tan powder (86% yield).
An attempt was made to purify this material by recrystallization from dry methanol, which failed. 90% methanol, 10% water was then tried, which
also gave a dark brown oil. I ended up reprecipitating with icewater, but the emulsion did not "curdle" like the first time and it remained white and
milky with some chunks of tan material at the bottom. An attempt at vacuum filtration did not separate the emulsion and the medium frit plate plugged
completely after a portion of "milk" was drawn through. DCM was added to the emulsion and stirred vigorously, but the "milk" remained. It was found
that adding a few grams of NaCl caused the emulsion to break and the aqueous phase to clear. The DCM phase was boiled down, transferred to a 50ml RBF
with stirbar and completely stripped of DCM under vacuum.
Disregard the above italics if repeating this preparation and simply transfer the crude 2-ethoxynaphthalene to a 50ml RBF with a small stirbar. The
crude material is vacuum distilled with a short path stillhead. An air condenser is suitable for this scale, but ~40C water should be used for any
larger amount.

The bulk of the material passes over at 170C (~30torr) with some forerun in the 160C range and is collected as a very slightly yellow oil that freezes
in the reciever to a crystalline solid. Yield is 6.31g (73% yield from 2-naphthol, 85% recovery) of 2-ethoxynaphthalene as a white crystalline solid
melting at 33-34C (uncorr. lit, 36-37C).

It has a powerful scent reminiscent of a more floral, sweeter version of methyl benzoate that is otherwise hard to put a finger on. At extremely high
concentrations (nose right up to the bottle) it seems to have a disagreeable fecal smell. This could be impurity, but it would not surprise me seeing
as how indole, which is floral at low concentrations is fecal at any decent concentration. Nerolin may activate the same receptors, but with different
affinities.
It might be possible to properly recrystallize the material at this point and improve the m.p, but I'm sick of working with it after the first
recrystallization failures.
[Edited on 10-26-13 by UnintentionalChaos]
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
Random
International Hazard
    
Posts: 1120
Registered: 7-5-2010
Location: In ur closet
Member Is Offline
Mood: Energetic
|
|
Nice work as always Magpie I love reading your experimental procedures.
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
Nice work, both of you!
Question: Why the specific warning for methanol? I'd be more weary of exposure to alkylbromides and iodides.
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Quote: Originally posted by vulture  |
Question: Why the specific warning for methanol? I'd be more weary of exposure to alkylbromides and iodides. |
No reason other than I am aware of the hazards of methanol. Not so much those for the halides. Thanks for the warning.
I think this is an interesting comparison of the results for two slightly different procedures by two different experimenters. Oil-outs are a bitch
and I think one is wise to go to distillation when they are encountered.
I placed my nerolin on a watch glass in my glassware cabinet. I am pleased by its pleasant smell every time I open the cabinet .
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Diachrynic
Hazard to Others
  
Posts: 226
Registered: 23-9-2017
Location: western spiral arm of the galaxy
Member Is Offline
Mood: zenosyne
|
|
2-Ethoxynaphthalene using ethanol and sulfuric acid
I want to present another alternative synthesis and workup for 2-ethoxynaphthalene, which avoids alkyl halides.
The preparation comes from an article by Gattermann from the late 19th century.[1]
Chemicals:
2.5 g 2-naphthol
2.5 mL absolute ethanol
1 g 98 % sulfuric acid
MTBE
Sodium hydroxide
Sodium sulfate for drying
For the pad:
Cyclohexane
Silica gel 60 A, 40-63 µm
Procedure:
The 2.5 g of 2-naphthol were dissolved in the ethanol (2.5 mL) in a 25 mL flask, then the 1 g of 98 % sulfuric acid was added carefully. This addition
is quite exothermic. A reflux condenser is added and the mix is refluxed for 4 hours by using an oil bath at 145 °C.
Then, the flask is allowed to cool a bit and the contents are dumped into 50 g of icewater, which is then extracted with 30 mL of MTBE in two
portions. (I'm sure diethyl ether would work just as well if not better.) The organic layers are combined.
15 g of sodium hydroxide was dissolved using 50 mL of water, left to cool and then used to wash the MTBE extract in two portions. Three phases are
observed: An aqueous bottom layer, above that a brown oil and above that a slightly brown MTBE layer. Only the top MTBE layer is retained. On standing
the brown oil solidifies into a glittering plate-like solid, it is 2-naphthol.
The MTBE is dried over anhydrous sodium sulfate, then removed in a simple distillation using a water bath. The residual liquid froze in the fridge.
Yield 2.19 g (73 %) of slightly brownish 2-ethoxynaphthalene.

Analysis:
A TLC (silica 60 F254, glass backed) was done using 9/1, v/v, n-hexane/ethyl acetate as the eluent. Visualized with 250 nm UV
(2-ethoxynaphthalene shows a blue fluorescence) and by staining with Hanessians stain (developed at 250 °C with a heatgun). Approximate Rf
values are 0.21 for 2-napthol and 0.70 for 2-ethoxynaphthalene. The product contains traces of 2-naphthol and another unknown impurity at an
Rf of about 0.29.

Left lane: 2-Naphthol.
Middle lane: The brown oil.
Right lane: Final MTBE extract before evaporation.
Between the lanes co-spots of the adjacent lanes.
Left image under 250 nm UV, right one after staining with Hanessians stain.
Further workup:
The product was padded with cyclohexane. A glass frit with a diameter of about 2 cm and a height of about 10 cm (called a "filter tube, according to
Allihn", example from Roth) was filled with a slurry of silica gel (60 A, 40-63 µm) in cyclohexane. This was put onto a filtration flask with a vacuum
pump attached. Then the product was dissolved in about 20 mL of cyclohexane, added onto the column, the solvent was run into the silica and it was
eluted using about 80 mL of cyclohexane. In the end the column was pulled mostly dry with the vacuum. All the brown and yellow impurities stay within
1 cm of the top, the filtrate is colorless. The cyclohexane is recovered by distillation, a vacuum is applied at the end to remove the last amount of
cyclohexane, the residual liquid freezes into a white solid in the fridge.
After drying in air, the white solid weighs 1.97 g (66 % based on 2-naphthol).

Discussion:
Washing the MTBE with sodium hydroxide is supposed to remove most unreacted 2-naphthol. If the 2-naphthol that precipitated out was included, the
yield based on unrecovered 2-naphthol would likely be considerably higher.
The padding eluent was chosed by suggestion of CorrosiveChemistry, who I'd like to thank here. I'm quite happy with how it turned out.
The original paper also mentioned the same method works for the methyl ether. In this case they heated to 125 °C, but they do mention that it is
beneficial to work "under the pressure of a small mercury column". So some slight pressure seems to help in the case of the methyl ether. Maybe it
could work without?
Literature:
[1] - L. Gattermann, Justus Liebigs Ann. Chem. 1888, 244, 29-76. https://doi.org/10.1002/jlac.18882440104
[Edited on 29-11-2023 by Diachrynic]
we apologize for the inconvenience
|
|
|
DraconicAcid
International Hazard
    
Posts: 4412
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Nice. I've made the methyl version by refluxing with methanol and sodium hydrogen sulphate, and I've added the ethyl bromide-based synthesis to my
organic lab. It works well. The purification is simplified by the fact that naphthol has very low solubility in dichloromethane.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
AvBaeyer
National Hazard
   
Posts: 655
Registered: 25-2-2014
Location: CA
Member Is Offline
Mood: No Mood
|
|
Diachrynic - It is quite possible that one of the lower Rf impurities is 1-ethyl-2-hydroxynaphthalene. Alkylation of 2-naphthol at the 1-position
under basic conditions is a known reaction.
DraconicAcid - Can you please post a procedure for your prep of 2-methoxynaphthalene using sodium bisulfate?
|
|
|
DraconicAcid
International Hazard
    
Posts: 4412
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Hmm. I know I've done it, but I just went through seven lab notebooks and can't find it. I need to be more organized.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4412
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Aha! Found the citation!
https://patents.google.com/patent/CN102285871A/en
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Metacelsus
International Hazard
    
Posts: 2543
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
This is a bit off topic, but it's my pet peeve when people write ß (the German double S) instead of β (the Greek letter).
Alzheimer's researchers who abbreviate amyloid-beta as Aß are making asses of themselves, see for example: https://pubmed.ncbi.nlm.nih.gov/11145990/
|
|
|
Texium
Administrator
       
Posts: 4665
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: Preparing to defend myself (academically)
|
|
Literally 
|
|
|
DraconicAcid
International Hazard
    
Posts: 4412
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
I finally found my notes from that reaction.
1.424 g 2-naphthol, 0.60 g sodium hydrogen sulphate monohydrate, and 1.5 mL of methanol were refluxed for 90 minutes. The mixture turned dark brown
(probably due to oxidation of the naphthol). The solution was cooled to room temperature, and 5 mL water and 2 mL 0.5 M potassium hydroxide solution
were added. This was extracted with 3 x 3 mL of dichloromethane.
The combined extracts were washed twice with 0.25 M potassium hydroxide solution (which did not diminish the dark colour of the solution), The
extract was then dried over sodium carbonate, and filtered through a plug of alumina (which removed much of the colour). The solvent was removed by
evaporation.
I know I checked it by mp and TLC, but didn't record those numbers in the right spot.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Fery
International Hazard
    
Posts: 1052
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
DraconicAcid great! Washing with KOH certainly got rid of unreacted naphthol. So you did a methylation without methylating agent like dimethylsufate
or methyl iodide... When I used dimethylsulfate the first methyl group reacted quickly and I wanted to push more product by forcing the reaction with
the second methyl group. The second methyl group required steam distillation to remove the product to push the equilibrium towards the product:
https://www.sciencemadness.org/whisper/viewthread.php?tid=15...
I further tried ethoxy https://www.sciencemadness.org/whisper/viewthread.php?tid=15... and allyloxy https://www.sciencemadness.org/whisper/viewthread.php?tid=15...
All the 3 above experiments had something different like solvent / base used.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4412
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
I'm not sure it's the KOH wash that's getting rid of the naphthol, but the use of dichloromethane (in which naphthol isn't very soluble) really helps.
I want to move away from chlorinated solvents, but it just works so damn well....
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
AvBaeyer
National Hazard
   
Posts: 655
Registered: 25-2-2014
Location: CA
Member Is Offline
Mood: No Mood
|
|
Questions about synthesis of 2-Ethoxynaphthalene using ethanol and sulfuric acid
Recently Diachrynic reported results (see above) on the acid catalyzed reaction of ethanol with 2-naphthol to afford 2-ethoxynaphthalene. A yield of
73% of initial product and 66% of purified product is reported. No melting point of the product is reported.
I have executed the same reaction under several conditions including that reported by Diachrynic and have never achieved a yield in excess of 35% of
crystalline 2-ethoxynaphthalene melting at 34-36 C. My reactions were all run using 5.0 g of 2-naphthol (mp 120-121 C), undenatured absolute ethanol
and 96% sulfuric acid (by titration). I use hexane to extract the product from the work up mixture. The crystalline product is isolated directly from
the hexane extract after a brine wash, drying, and evaporation of the hexane. I can recover about 85% of the unreacted 2-naphthol which is extremely
difficult to filter.
The basic procedure that I have followed is that reported by Stork for the synthesis of 2-methoxynaphthalene. Following that procedure at the 5.0 g
scale and using a slightly modified work up, I routinely achieve yields of at least 85% of 2-methoxynaphthalene. When using ethanol, the molar amounts
of ethanol and sulfuric acid are adjusted to match those when using methanol per the stoichiometry in the reference.
Modified work up: The hot reaction mixture is poured into 1 M NaOH using about 15 times the volume of alcohol used in the reaction. The final pH
should be about 12 (paper). A few chips of ice are added to cool the mixture which results in crystallization of the product. This is not filtered but
extracted 3 times with hexane each of which is about 1/3 the volume of the aqueous portion. The hexane extracts are washed with water, brine then
dried over sodium sulfate. The hexane is then removed by distillation. Residual solvent is removed at aspirator pressure during which time the product
crystallizes. Acidification of the aqueous layer precipitates unreacted 2-naphthol.
1. What is the melting point of Diachrynic’s product?
2. What type of ethanol was used? What is the denaturant, if any? I ask this to determine if methanol was used to denature the ethanol used. The
methoxy and ethoxy compounds are not readily distinguishable by TLC.
Reference: G. Stork, J. Am. Chem. Soc., 69, 576 (1947)
Any discussion?
AvB
[Edited on 11-12-2023 by AvBaeyer]
|
|
|
| Pages:
1
2 |