Chemundergrad
Harmless

Posts: 4
Registered: 19-5-2012
Member Is Offline
Mood: No Mood
|
|
Organic Chem, Triphenyl Methanol.
I have a question to do with Triphenyl Methanol reacting with mineral acids, on my lab work at uni. Apparently there is a reactive bright
yellow reactive intermediate. It asked for me to eplain this observation and identify the intermediate.
I was thinking of something alonG the lines of this. Is it possible?
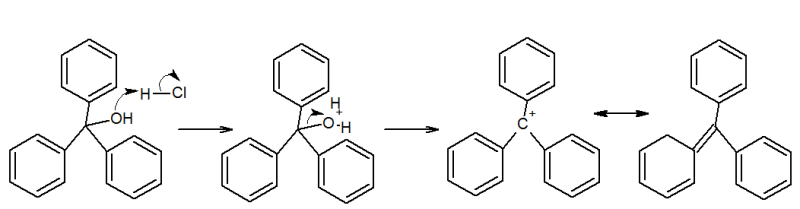
Thanks Alex
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
http://en.wikipedia.org/wiki/Triphenylmethanol
|
|
|
Chemundergrad
Harmless

Posts: 4
Registered: 19-5-2012
Member Is Offline
Mood: No Mood
|
|
Thankyou for the reply, and just want to state I realise I have missed a + charge on the second resonance form.
|
|
|
unionised
International Hazard
    
Posts: 5126
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
http://en.wikipedia.org/wiki/Carbocation
might help too.
|
|
|
Chemundergrad
Harmless

Posts: 4
Registered: 19-5-2012
Member Is Offline
Mood: No Mood
|
|
Thanks both of you for the help, I guess I was along the right track minus the added resonance form.
|
|
|