| Pages:
1
2
3
..
13 |
Polverone
Now celebrating 21 years of madness
        
Posts: 3186
Registered: 19-5-2002
Location: The Sunny Pacific Northwest
Member Is Offline
Mood: Waiting for spring
|
|
one little problem
Glass, fibrous or otherwise, is going to dissolve in molten NaOH. Are you an engineer by day, Orgy? You seem to have quite the love of Nifty
Apparatus.
|
|
|
Organikum
resurrected
    
Posts: 2342
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: frustrated
|
|
me stupid !
Of course you are right Polverone - I reread where I thought to have read about a porous fritte of glass and it says not glass but quartz and it is
told in relation with lithium electrolysis.
- I looked the properties of quartz up and the resistance against alkali seems to be very low? Oha!
Well I have no quartz, doesn´t matter....
As iron seems to work well I suppose steelwool to be the material of choice - perhaps it is possible to press it for a semisintered consistence?
A engineer by day? No, by daytime I am sleeping for I would decompose in sunlight......
But yes nifty apparatus are more my turf as formulas and reagents with unspeakable names. But my love to electrochemistry has cooled out since I
discovered the world of pyrolysis and - new - had a look in photochemistry and ozone.
Ah! Deadly new flames on the horizon! UV! Ozone!
Electrochemistry for organic synthesis is diappointing I learned. To much effort, this makes no sense exept if used for preparation/regeneration of
reagents like manganeseIII compounds (in situ so any possible) . Ozone has also quite restricted use - in KMnO4 synthesis perhaps? But photochemistry
is like the hot tube! Endless possibilities.
photoreactor:
(there are designs available which are not as demanding in glassware - the quartztubes alone are very expensive here)
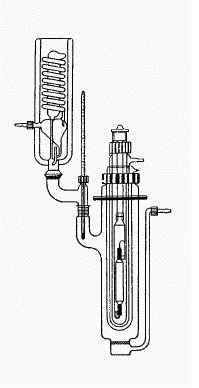
|
|
|
Polverone
Now celebrating 21 years of madness
        
Posts: 3186
Registered: 19-5-2002
Location: The Sunny Pacific Northwest
Member Is Offline
Mood: Waiting for spring
|
|
please share
Pyrolysis? Some months back madscientist told me with great excitement of things he was learning about pyrolytic processes from some book or other.
Unfortunately, he doesn't do book scans, so I never got to look at what sort of amazing things he was reading about.
I'd also be interested in hearing more about photochemistry. It seems that it won't be as affordable as pyrolysis and tube furnaces, due to
all the glass (quartz?) needed and the expense of high output UV lamps. Still, I am always interested in lesser-known synthetic processes, especially
those that might be realizable by an amateur.
No doubt it would take some time to write down all you have learned, but maybe you can share the documents that have most excited/enlightened you.
|
|
|
BromicAcid
International Hazard
    
Posts: 3266
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
I posted this on sci.chem awhile ago and one of the regular's there said this process sounds dangerous, the information comes from V.A. Plotnikov
and Z.A. Yankelevich, Mem. Inst. Chem. Acad. Sci. Ukrain (USSR) 9, 420-33 (1936) ; Chem. Zentr. (1937), I, 3450-1
"... the alkali chloride of bromide is fused with aluminum chloride or bromide and the fused mass dissolved in nitrobenzene and electrolyzed. By
this method, lithium, sodium, potassium, and rubidium can be deposited on the cathode. The alkali ion serves as the cation, and the aluminum appears
in the complex anion."
I always thought that way was interesting, here's another.
"The mobility of sodium ions in a soda-lime-silica glass at elevated temperatures is fairly high; if an evacuated bulb of such a glass is dipped
into molten sodium nitrate and electrolysis is brought about by bombarding the inside of the bulb with electrons, the circuit being completed with an
electrode in the sodium nitrate, then metalic sodium appears in the bulb."
This came from an eBook that I have about lab glass, I can personally vouch for this reaction working although I cannot give volatages, and best of
all sodium nitrate melts at a realatively low temperature and is safer then a molten chloride or expecially the hydroxide.
|
|
|
Theoretic
National Hazard
   
Posts: 776
Registered: 17-6-2003
Location: London, the Land of Sun, Summer and Snow
Member Is Offline
Mood: eating the souls of dust mites
|
|
The McGraw-Hill "Handbook of inorganic industrial chemicals" says Na reacts with NaOH between 300 and 385 degrees. Don't know why it
doesnt go above that.
Check out this. Sodium acetate is soluble in ether, and I'm sure it's ionized, thus electrolytic extraction of sodium can actually be
carried out at room temperature! There were other solvents, but they all would react with sodium.
4CH3COONa=>4Na+2(CH3CO)2O+O2
(CH3CO)O+Na2CO3=>2CH3COONa+CO2
 
|
|
|
Madog
Hazard to Others
  
Posts: 221
Registered: 20-5-2002
Location: USA
Member Is Offline
Mood: lysergic
|
|
last night i took some dry sodium acetate and added it to some ether in my lab, the fumes were getting me high... anyways, i swirled it around and
warmed it and then, not really seeing any disolve, i just poured off the ether into another beaker, hopeing some was disolved. i proceeded to hook up
2 electrodes in it with a car batery charger, it didnt conduct. :-(
Most people outgrow their pyro tendencies, we are the ones who\'s tendencies outgrew us.
|
|
|
madscientist
National Hazard
   
Posts: 962
Registered: 19-5-2002
Location: American Midwest
Member Is Offline
Mood: pyrophoric
|
|
Are you sure the sodium acetate was truly dry? It might have appeared so while actually being a hydrate (probably
NaCH<sub>3</sub>COO*3H<sub>2</sub>O).
I weep at the sight of flaming acetic anhydride.
|
|
|
Theoretic
National Hazard
   
Posts: 776
Registered: 17-6-2003
Location: London, the Land of Sun, Summer and Snow
Member Is Offline
Mood: eating the souls of dust mites
|
|
http://avogadro.chem.iastate.edu/MSDS/NaOAc-3H2O.htm
Looks like anhydrous acetate is insoluble in ether  , but the hydrate is
soluble. , but the hydrate is
soluble.
On dissolution, the hydrate is likely to give off water as another layer, but water is a few percent soluble in ether and thus will have to be
removed-CaCl2 or CaSO4 or something...
[Edited on 29-9-2003 by Theoretic]
|
|
|
Theoretic
National Hazard
   
Posts: 776
Registered: 17-6-2003
Location: London, the Land of Sun, Summer and Snow
Member Is Offline
Mood: eating the souls of dust mites
|
|
BUT!
"General
Synonyms: sodium acetate anhydrous
Molecular formula: CH3COONa
CAS No: 127-09-3
EC No: 204-823-8
Physical data
Appearance: white crystals or powder
Melting point: 58 C
Boiling point: 120 C
Vapour density:
Vapour pressure:
Specific gravity: 1.528
Flash point:
Explosion limits:
Autoignition temperature: 600 C
Water solubility: substantial"
Just the thing for molten electrolysis!
|
|
|
rikkitikkitavi
Hazard to Others
  
Posts: 192
Registered: 17-6-2002
Member Is Offline
Mood: No Mood
|
|
dont think it would help to dissolve sodium acetate in ether , since it is not ionized in this solution, hence non conductive.
some solvents can dissolve ionic components into ions , but not all. It is just a matter of finding the right solvent where Na+ + e- => Na is going
to take place at the cathode. Aluminium has been electroplated in some water solvents , but I dont remember what they where .
/rickard
|
|
|
Theoretic
National Hazard
   
Posts: 776
Registered: 17-6-2003
Location: London, the Land of Sun, Summer and Snow
Member Is Offline
Mood: eating the souls of dust mites
|
|
Not ionized? You mean floating around in clusters of anions and cations? Why would it dissolve then if the energy of solvatation isn't released?

|
|
|
rikkitikkitavi
Hazard to Others
  
Posts: 192
Registered: 17-6-2002
Member Is Offline
Mood: No Mood
|
|
exactly!
it is the molecule itself that is solvatized.
Also probably because sodium acetate is not a strong ionic compound as f e x sodium chloride.
If there where free ions , there would be conductivity, since free ions always move in an electrical field, and that will lead to a current flow =
conductivity. So I simply deduct that sodium acetate is not ionized in ether.
/rickard
|
|
|
BromicAcid
International Hazard
    
Posts: 3266
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
This may prove to be useful information. Recently I was researching a way to lithium metal production and came across two interesting things
involving electrolysis. Electrolysis of a solution of lithium chloride in pyridine yields lithium metal. You can also electrolyze a solution of
lithium chloride in ethanol and actually get lithium metal. One source said the reaction of lithium with chilled ethanol is negligible and
electrolysis is possible after a certain saturation of lithium in the solution is achieved. I highly doubt that any alcohol could be used for
electrolysis of a sodium salt but maybe pyridine. Also there was an article I posted back when the mentioned electrolysis of sodium chloride with
aluminum chloride in nitrobenzene. Although obtaining nitrobenzene can be expensive.
|
|
|
Marvin
National Hazard
   
Posts: 995
Registered: 13-10-2002
Member Is Offline
Mood: No Mood
|
|
Ive been thinking a lot about organic solvents, and the problem thats been plagueing me, is that as soon as you have a useful cathode reaction for
produce sodium you then have to worry about anode reactions.
In ethanol things might be workable, but what is being produced at the anode, and how are these products kept seperate from the sodium compartment
without killing the electrolysis. Furthurmore for me if its destorying the solvent at the same rate its producing sodium and this is something exotic
like pyirdine or nitrobenzene, that for me makes it unworkable.
One phrase bothers me, what is actually meant by the solution becoming saturated in lithium?
|
|
|
Mr. Wizard
International Hazard
    
Posts: 1042
Registered: 30-3-2003
Member Is Offline
Mood: No Mood
|
|
aluminum plating in water
| Quote: | Originally posted by rikkitikkitavi
dont think it would help to dissolve sodium acetate in ether , since it is not ionized in this solution, hence non conductive.
some solvents can dissolve ionic components into ions , but not all. It is just a matter of finding the right solvent where Na+ + e- => Na is going
to take place at the cathode. Aluminium has been electroplated in some water solvents , but I dont remember what they where .
I was filling some balloons with hydrogen by reacting aluminum electrical cable with lye and water. The reaction wasn't going as fast as I
wanted, so I added some copper wire scrap to provide contact with the aluminum and form an electric couple and speed up the reaction. I didn't
notice any difference in hydrogen production, but on emptying the bottle of its liquid, I found the copper was plated with aluminum! I never tried it
again, but your comment made me remember it. Maybe the nascent hydrogen had something to do with it?
/rickard |
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
not electrolytic - but back to making sodium!
I'm fascinated by the Castner process and want to try it sometime but I'm wondering if there isn't an easier way to make sodium. My
baby chemistry book states that sodium was originally made by the reduction of sodium carbonate to Na and CO using carbon and heat. I did some
thermodynamic calculations to see what temperature would be needed to get a spontaneous reaction (negative free energy change). This came out about
1200 deg C. Sodium boils at 892 deg C so I don't think you'd want to go much higher than the melting point of sodium carbonate which is
851. Evolution of CO would help drive the reaction. An argon or N2 blanket would be needed to protect the floating liquid Na. I realize this would
take a muffle furnace and an argon bottle, but electrolysis doesn't sound real easy either.
Incidentally, has anyone tried a standard car battery charger (6v/12v) as a power supply for electrolysis?
|
|
|
Polverone
Now celebrating 21 years of madness
        
Posts: 3186
Registered: 19-5-2002
Location: The Sunny Pacific Northwest
Member Is Offline
Mood: Waiting for spring
|
|
alkali metals sans electricity
For the carbothermic production of potassium as practiced in the 19th century, see http://bcis.pacificu.edu/~polverone/muspratt2/c-0724.html and the following page. See also http://bcis.pacificu.edu/~polverone/muspratt2/c-0894.html for the modifications pertinent to making sodium.
Lithium carbonate can be reduced with aluminum powder at a lower temperature (in vacuum or under inert gas blanket) to yield a mixture of lithium and
carbon, IIRC.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
carbothermic metal production
Thank you Polverone for those most interesting references to the pioneer making of Na and K. What a thrill it must have been to first lay eyes on
those metals! We should always remember that these honored men (Davy, Gay-Lussac, etc)were the "Mad Scientists" of their day.
It appears that indeed it does take a very high temperature to drive these reactions as they are talking about a "white hot" reaction
vessel.
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
How about the electrolysis of Na-salts of long-chain aliphatic carboxy acids? such as sodium stearate, oleate, palmitate, or even proprionate,
butyrate etc?
According to Kabooms post in another thread ( http://www.sciencemadness.org/talk/viewthread.php?tid=1050 ) ,the Kolbe reaction should take place. But what happens at the Kathode?? Na might
actually sequester there!
Comments anyone?
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
Theoretic
National Hazard
   
Posts: 776
Registered: 17-6-2003
Location: London, the Land of Sun, Summer and Snow
Member Is Offline
Mood: eating the souls of dust mites
|
|
In a non-aqueous, non-ionizable solvent nothing else can happen unless there's foreign cations in the solution.  
I suggest ether, acetone or alcohol. Ass cheap, non-ionisable and dissolve the salts you want to use.
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
I guess I should have been clearer, I actually meant electrolysis of sodium carboxylic acid salts in their *molten*states, rather than dissolving it
in EtOH etc.
For instance, sodium stearate should be liquid at 80 deg C. ...but I doubt the conductivity is great. However, I am sure the conductivity gets better
the shorter the chainlenght... anyway, this is what I was thinking of 
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
KABOOOM(pyrojustforfun)
Hazard to Others
  
Posts: 254
Registered: 12-10-2002
Location: Iran (pseudoislamic dictatorship of)
Member Is Offline
Mood: exuviating!
|
|
in molten state it probably produces a mix of R-R , RCOO-R , CO<sub>2</sub> & leaves
Na<sub>2</sub>CO<sub>3</sub> behind.
edit: just found what ya meant. carbonate wont form due to lack of water. you may get Na @ cathode.
it really worths trying
[Edited on 27-11-2003 by KABOOOM(pyrojustforfun)]
|
|
|
Theoretic
National Hazard
   
Posts: 776
Registered: 17-6-2003
Location: London, the Land of Sun, Summer and Snow
Member Is Offline
Mood: eating the souls of dust mites
|
|
Molten carboxylic acid salts is what I proposed long ago.  Sodium acetate, to be
specific. It melts at 324 C, so a hotplate will do the job, never mind a gas flame. Another MSDS states the mp as being 58 C, but 324 C is supported
by two MSDS's, while the 58 C figure only by one. NEVER TRUST MSDS'S ON TEMPERATURES (this is not my first experience of MSDS's not
agreeing with each other and being self-contradictory - e.g. in two MSDS's the mp of urea is stated as 133 C... ...and the bp as 135 C!). I do
agree that the longer the hydrocarbon chain, the lower the mp, but the viscosity is likely to increase as well, thus slowing down gas bubbles that
escape and, with very long chains, turning into froth upon electrolysis. Sodium acetate, to be
specific. It melts at 324 C, so a hotplate will do the job, never mind a gas flame. Another MSDS states the mp as being 58 C, but 324 C is supported
by two MSDS's, while the 58 C figure only by one. NEVER TRUST MSDS'S ON TEMPERATURES (this is not my first experience of MSDS's not
agreeing with each other and being self-contradictory - e.g. in two MSDS's the mp of urea is stated as 133 C... ...and the bp as 135 C!). I do
agree that the longer the hydrocarbon chain, the lower the mp, but the viscosity is likely to increase as well, thus slowing down gas bubbles that
escape and, with very long chains, turning into froth upon electrolysis. 
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
Lol theoretic, sorry, I must have overlooked this. You didnt specifically mention Na salts of long chain aliphatic carboxylic acids though. I
wasn't thinking of Naacetate, as indeed this has been covered. THe strangely low melting point of Naacetate probably refers to the decahydrate,
the 'anhydrous' being a mistake. This one is obviously useless.
Hmm, 324 deg and a hotplate? that gotta be a damn hot one at that!! you are speaking of getting to lead melting temperatures!
Anyway, I think I may try this, with palmitate or stearate.... but also, maybe Na-phenolate!!! (NaOC6H5).
pz 
PS yes, kaboom that's what I thought too, carbonate shouldnt really form at all! It should be at the anode, unless some strange electrochemical
reactions are happening that I am not aware of 
[Edited on 27-11-2003 by chemoleo]
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
Organikum
resurrected
    
Posts: 2342
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: frustrated
|
|
No
the iron screen is not absolutely necessary in a NaOH-cell. In a smaller setup the use of higher voltages is favorable anyways for to provide a better
heating by the electricity so a wider distance between the electrodes doesnt hurt at all.
The concrete is for mechanical/chemical resistance mainly - the glasswool/stonewool is for the insulation. The bottom is left uninsulated as this
might be a bad idea - I usually apply the propane/petroleum burner at this place ya know?
|
|
|
| Pages:
1
2
3
..
13 |