Teen Chemist
Harmless

Posts: 42
Registered: 27-4-2012
Member Is Offline
Mood: Effervescent
|
|
Interesting molecules
I thought it would be cool to create a thread for interesting molecules.
This applies to molecules that have interesting/cool/unusual:
1. Structures
2.Names
3.Appearances
4.Properties
5.Other
I know there are other threads like this such as http://www.sciencemadness.org/talk/viewthread.php?tid=17747 but this is ment to put them all together.
[Edited on 25-7-2012 by Teen Chemist]
|
|
|
achem500
Harmless

Posts: 31
Registered: 21-7-2012
Location: South Carolina, USA
Member Is Offline
Mood: Curious
|
|
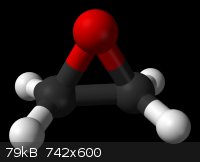
Ethylene Oxide. It looks pretty cool and dangerous 
[Thanks to Wikipedia for the picture]
|
|
|
kristofvagyok
National Hazard
   
Posts: 659
Registered: 6-4-2012
Location: Europe
Member Is Offline
Mood: No Mood
|
|
I would suggest azulene for this "list". It is a pretty interesting molecule. In the lab where I work, someone had a research about polyfunctionated
azulene compounds and she had really good results.
The structure of naphthalene and azulene:

They are both aromatic and they both consist of 10 carbon and 8 hydrogen atom, the only difference that naphthalene is more stable, so if the azulene
is exposed to strong light or heat, it will isomerise "back" to naphthalene.
Pure azulene looks like this:

The rule for the colors of the azulenes: if it has electron withdrawing groups, it is much brighter (red, yellow), if it has electron releasing
groups, it is darker (green, brown ect.).

And also an interesting fact about it: it has a similar odor to naphthalene and it sublimes, so never store it in a "normal" vessel!
More azulene related pictures and some info about it:
http://labphoto.tumblr.com/tagged/azulene
I have a blog where I post my pictures from my work: http://labphoto.tumblr.com/
-Pictures from chemistry, check it out(:
"You can’t become a chemist and expect to live forever." |
|
|
99chemicals
Hazard to Others
  
Posts: 174
Registered: 24-3-2012
Location: In the Octet
Member Is Offline
Mood: No Mood
|
|
Crown Ether
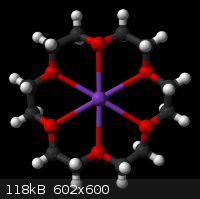
I found the picture from Wikipedia.
|
|
|
Vargouille
Hazard to Others
  
Posts: 380
Registered: 16-4-2012
Member Is Offline
Mood: No Mood
|
|

Copper salicylate. Water soluble, and has some use as an anti-inflammatory.
|
|
|
Teen Chemist
Harmless

Posts: 42
Registered: 27-4-2012
Member Is Offline
Mood: Effervescent
|
|
Buckminsterfullerenes aka buckeyballs
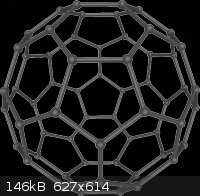
Thanks to wikipedia for pic.
|
|
|
FrankWayne
Unregistered
Posts: N/A
Registered: N/A
Member Is Offline
|
|
For a molecular researchers, a nice saying-
"An aeroplane is always safe on ground but it is not made for that" , so to achieve great heights it needs to take risks.
Did anyone see the molecular structure of diamond ? It is quite interesting !
|
|
|
White Yeti
National Hazard
   
Posts: 816
Registered: 20-7-2011
Location: Asperger's spectrum
Member Is Offline
Mood: delocalized
|
|
Carotenoids are pretty nice. Their conjugated double bonds give these compounds nice colours and potent antioxidant activity. Speaking of which,
Staphylococcus aureus produces Staphyloxanthin a potent antioxidant, which gives it the ability to survive in environments with high
oxidative stress.
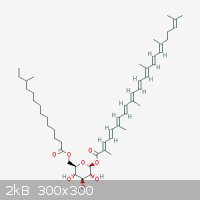
"Ja, Kalzium, das ist alles!" -Otto Loewi
|
|
|
Teen Chemist
Harmless

Posts: 42
Registered: 27-4-2012
Member Is Offline
Mood: Effervescent
|
|
Good work so far anyone got anything more exotic.
|
|
|
Adas
National Hazard
   
Posts: 711
Registered: 21-9-2011
Location: Slovakia
Member Is Offline
Mood: Sensitive to shock and friction
|
|

TEMPO - stable free radical
Rest In Pieces!
|
|
|
DoctorOfPhilosophy
Hazard to Others
  
Posts: 130
Registered: 12-6-2012
Location: Ontario, Canada
Member Is Offline
Mood: enthralled
|
|
Helium compounds all fall into the extreme chemistry catagory, my favorites are the hydride and HgHe10. Apparently the former is "the
strongest Brønsted acid known, and therefore can exist only in isolation, as it will protonate any molecule or counteranion it comes into contact
with." There is even a HeNe+ polyatomic ion.
See:
http://en.wikipedia.org/wiki/Helium#Compounds
http://en.wikipedia.org/wiki/Neon#Compounds
|
|
|
triplepoint
Hazard to Others
  
Posts: 127
Registered: 11-4-2012
Location: U.S.
Member Is Offline
Mood: in equilibrium
|
|
kristofvagyok:
The azulenes look especially intriguing to me, but the notes on your tumblr site tell me i won't be trying this myself in the near future.
|
|
|
Lithium
Hazard to Others
  
Posts: 104
Registered: 25-2-2012
Location: Australia
Member Is Offline
Mood: Thinking!
|
|
 6-acetyl-2,3,4,5-tetrahydropyridine 6-acetyl-2,3,4,5-tetrahydropyridine
supposedly is the culprit for the smell of bread, which, I must say, is one of my favourite smells
Li
|
|
|
Endimion17
International Hazard
    
Posts: 1468
Registered: 17-7-2011
Location: shores of a solar sea
Member Is Offline
Mood: speeding through time at the rate of 1 second per second
|
|
Platonic hydrocarbons are cool.
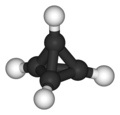  
Few years ago, I've been playing with ChemSketch and made them in the 3D simulator, having no idea they actually existed (except tetrahedrane) or what
were their names.
The polyol derivatives might be very interesting compounds.
|
|
|
zoombafu
Hazard to Others
  
Posts: 255
Registered: 21-11-2011
Location: U.S.
Member Is Offline
Mood: sciencey
|
|
Quote: Originally posted by Lithium  |
6-acetyl-2,3,4,5-tetrahydropyridine
supposedly is the culprit for the smell of bread, which, I must say, is one of my favourite smells |
The smell of bread is amazing. I totally agree with you.
|
|
|
kristofvagyok
National Hazard
   
Posts: 659
Registered: 6-4-2012
Location: Europe
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Endimion17  | Platonic hydrocarbons are cool.
  
Few years ago, I've been playing with ChemSketch and made them in the 3D simulator, having no idea they actually existed (except tetrahedrane) or what
were their names.
The polyol derivatives might be very interesting compounds. |
I know someone who synthetises cubane derivatives. Shitty chemistry with a lot steps with really low yields. And they are not as useful how they look.
Also: the polyols of these molecules would be extremely instable, maybe the cubane 1,4 diarboxylic acid is one of the most stable, but it is still not
the best. If any transition metals are present near to the cubane skeleton (even in ppm concentrations) it will isomerize to cuneane.
And for the explosive fans: octanitrocubane is highly hygroscopic, so not just the price of that says that it is not an optimal explosive.
I have a blog where I post my pictures from my work: http://labphoto.tumblr.com/
-Pictures from chemistry, check it out(:
"You can’t become a chemist and expect to live forever." |
|
|
BromicAcid
International Hazard
    
Posts: 3253
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Aluminum tetraazidoborate
Al[B(N3)4]3
"Mellor, 1967, Vol. 8, Suppl. 2, 2"
"A very shock sensitive explosive, containing nearly 90 wt% of nitrogen." From Reactive Chemical Hazards and referenced by me in this thread:
http://www.sciencemadness.org/talk/viewthread.php?tid=1172
|
|
|
maxpayne
Hazard to Self
 
Posts: 78
Registered: 15-11-2011
Member Is Offline
Mood: No Mood
|
|
Leuco dyes are quite interesting.
http://en.wikipedia.org/wiki/Leuco_dye

|
|
|
Hexavalent
International Hazard
    
Posts: 1564
Registered: 29-12-2011
Location: Wales, UK
Member Is Offline
Mood: Pericyclic
|
|
Arsole - the cyclic organoarsenic compound - just because of it's name
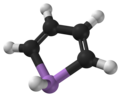
[Edited on 30-7-2012 by Hexavalent]
"Success is going from failure to failure without loss of enthusiasm." Winston Churchill
|
|
|
arsphenamine
Hazard to Others
  
Posts: 236
Registered: 12-8-2010
Location: I smell horses, Maryland, USA
Member Is Offline
Mood: No Mood
|
|
This may be a trifle Zen but I think any compound is interesting if you look at it hard enough. Example: singlet vs. triplet O2.
NB: tetrahedrane has yet to be synthesized although a few fully substituted ones (t-butyl and tetramethyl silyl) are out there.
|
|
|
feacetech
Hazard to Others
  
Posts: 163
Registered: 12-2-2007
Member Is Offline
Mood: No Mood
|
|
CHB11H11-
Used to make solid super acids
Interesting article that can explain it in more depth
Attachment: strongest acid.pdf (1MB)
This file has been downloaded 683 times
[Edited on 26-8-2012 by feacetech]
|
|
|
Teen Chemist
Harmless

Posts: 42
Registered: 27-4-2012
Member Is Offline
Mood: Effervescent
|
|
Solvatochromic dyes are compounds that change colors in various solvents.
http://en.wikipedia.org/wiki/Solvatochromism
A good example is Reichardt' s Dye.
[Edited on 2-9-2012 by Teen Chemist]
|
|
|