99chemicals
Hazard to Others
  
Posts: 174
Registered: 24-3-2012
Location: In the Octet
Member Is Offline
Mood: No Mood
|
|
Essential Oil Extraction
I would like to do a extraction of the citroella oils from citroella cented geranium leaves via distillation. Can somebody show me how to setup the
appuratus.
I have various sizes of RBFs (all one neck)
Ghram and west condesers
Vacuum take off adapter (no vacuum pump)
3 way adapter
1 themometer and adapter
Sep funnel (not pressure equalized)
Clasian adapter
Gas adapter/ vacuum adapter
Various solvent and alcohols
I know the basic idea of the prosess. You have to boil off the oils and collect them. But can you do that with a simple distillation setup?
[Edited on 6-5-2012 by 99chemicals]
|
|
|
Hexavalent
International Hazard
    
Posts: 1564
Registered: 29-12-2011
Location: Wales, UK
Member Is Offline
Mood: Pericyclic
|
|
There are several ways to do extractions, but the most common (and efficient!) seems to be steam distillation.
There are several ways in which to set up the apparatus for this, but here are the main ones;

where the steam is introduced directly into the material,

where hot water is added slowly, allowed to boil fully in the flask, convert to steam and take the oils with it that way,
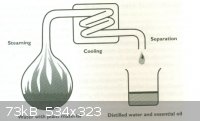
where water is just added straight to the sample in one go and distilled off.
A 'solvent distillation head' can also be used here, often to quite satisfactory results.
Steam distillation is often favoured as it protects temperature sensitive materials like natural aromatic compounds.
Many organic compounds tend to decompose at high sustained temperatures. Separation by normal distillation would then not be an option, so water or
steam is introduced into the distillation apparatus. By adding water or steam, the boiling points of the compounds are depressed, allowing them to
evaporate at lower temperatures, preferably below the temperatures at which the deterioration of the material becomes appreciable. If the substances
to be distilled are very sensitive to heat, steam distillation can also be combined with vacuum distillation; but obviously this is not suitable for
you, but TBH in 99% of cases it really isn't necessary. After distillation the vapors are condensed as usual, usually yielding a two-phase system of
water and the organic compounds, allowing for simple separation, either by drawing off one of the layers or using a sep funnel. If the layers do not
want to form a biphasic mixture on their own, then you can often get the oil out from my experiences by adding saturated NaCl to the mixture and
shaking, or doing a solvent extraction with something like DCM, EtOAc etc., tapping off the appropriate layer and then evaporating off the solvent to
yield the oil.
You may find this video useful, where a guy extracts geranium oil as an example of steam distillation. He uses a solvent distillation head as
discussed previously, followed by separation in a sep funnel; http://www.youtube.com/watch?v=gDojS7PWWPY
------------------------------------------------------------------------------------------
EDIT(woelen): Changed image link from allaboutaromatherapy.com. Reason of this change is that allaboutaromatherapy.com contains malware and may infect
your PC.
[Edited on 4-10-12 by woelen]
"Success is going from failure to failure without loss of enthusiasm." Winston Churchill
|
|
|
99chemicals
Hazard to Others
  
Posts: 174
Registered: 24-3-2012
Location: In the Octet
Member Is Offline
Mood: No Mood
|
|
Would this setup work? I will use the sep funnel to add more water when nessesary.
What would be a good setup to heat the big flask? I have a hot plate. Would that with an oil bath and a thermometer work? Just keep the oil below
120oc.

|
|
|
Hexavalent
International Hazard
    
Posts: 1564
Registered: 29-12-2011
Location: Wales, UK
Member Is Offline
Mood: Pericyclic
|
|
I can't see why that wouldn't work, just be certain to make the extract small enough to get into and out of the flask without difficulty; I've seen
people have great trouble when they've put things that are too big into flasks, and sometimes the act of removing them breaks the glass.
An oil bath would probably suffice, although a proper heating mantle is best. Just be sure not to heat it too quickly and, if you have a magnetic
stirrer, add a paperclip to the oil and stir slowly. I think aonomus has a video on it on YouTube; the slow motions help heat the oil evenly and more
quickly.
Additionally, don't forget not to put a stopper on the addition funnel; if you do, the pressures caused will allow nothing to fall from the funnel,
even with the tap wide open.
"Success is going from failure to failure without loss of enthusiasm." Winston Churchill
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
If you start off with enough water in the RBF you may not need to ever add any water during the distillation (distill fairly slowly). If you're gonna
use a sep funnel to add water, a pressure equalised sep funnel is recommendable to prevent steam from rising through the sep funnel's stopcock when
you open it.
Nice set up, though! 
|
|
|
99chemicals
Hazard to Others
  
Posts: 174
Registered: 24-3-2012
Location: In the Octet
Member Is Offline
Mood: No Mood
|
|
I setup my apperatus. I am using citrenilla scented geraniums. I do not have any mint right now and I am just doing a proof of concept.
Need to eat something and plug it in!

|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
I think your receiver RBF might be a tad small: plants do not contain large amounts of essential oils, you'll be receiving quite a bit of water.
Good luck!
[Edited on 5-6-2012 by blogfast25]
|
|
|
99chemicals
Hazard to Others
  
Posts: 174
Registered: 24-3-2012
Location: In the Octet
Member Is Offline
Mood: No Mood
|
|
If the reciving flask fills up then I will empty it into a beaker.
Nobody noticed that my condenser was upside down. I realized as soon as I started to heat. I was able to fix it.
Its been around 15 minutes of boiling and it is more refluxing than distilling. The condensation front has reached the top of the thermometer but, it
has not gone into the condenser yet. I put aluminum foil around the clasian and the 3 way. Homefully the insulation will help the distillation.
|
|
|
99chemicals
Hazard to Others
  
Posts: 174
Registered: 24-3-2012
Location: In the Octet
Member Is Offline
Mood: No Mood
|
|
UPDATE
The foil seems to have helped decrease reflux. I am now getting cloudy liquid in the reciving flask. The temparature in the still head is around 98
celcius.
|
|
|
Hexavalent
International Hazard
    
Posts: 1564
Registered: 29-12-2011
Location: Wales, UK
Member Is Offline
Mood: Pericyclic
|
|
Report back and tell us how it went!
Be careful about the receiver getting too heavy for the little plastic Keck clip to hold it all though.....there is nothing worse than have your
product fall and the flask break, and mind not to use too much grease...you really only do need to tiniest amount towards the top of the joint
"Success is going from failure to failure without loss of enthusiasm." Winston Churchill
|
|
|
Endimion17
International Hazard
    
Posts: 1468
Registered: 17-7-2011
Location: shores of a solar sea
Member Is Offline
Mood: speeding through time at the rate of 1 second per second
|
|
If the chemicals that are being distilled aren't very poisonous, or air-reactive, grease is needed only at the joints where vapor is expected.
They aren't neccessary after the condenser.
Mind that you'll have to remove the distillate somehow, and the easiest way is to pour it out, which will dissolve some of the grease and carry it
along.
Your setup is correct, however sometimes a real steaming out is preferred, as in the first photo of this thread.
I'll be glad to hear about the yields.
Hexavalent, don't worry about the clip, it's designed to hold such small weights. Even if the small receiving flask was full of mercury, it wouldn't
fall off, if not damaged.
|
|
|
99chemicals
Hazard to Others
  
Posts: 174
Registered: 24-3-2012
Location: In the Octet
Member Is Offline
Mood: No Mood
|
|
There is my insulated setup. When the collecting flask got half full I emptied it into a beaker. You can see some drops of oil floating on the
surface.
 
The thermometer in the oil is 160 and the 3-way is 103 celcius
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Yep, mucho water, poco oil: you're probably doing it right! Any capability of identifying what you're carrying over, post-distillation?
|
|
|
99chemicals
Hazard to Others
  
Posts: 174
Registered: 24-3-2012
Location: In the Octet
Member Is Offline
Mood: No Mood
|
|
I know I have some citronella that came over. I put in a bunch of leaves from a citronella geranium. I have it in a sep funnel now with some salt
water. I am going to let that settle until the water phase goes clear then pour of the water.
The whole room smells like the citroella oil.
|
|
|
99chemicals
Hazard to Others
  
Posts: 174
Registered: 24-3-2012
Location: In the Octet
Member Is Offline
Mood: No Mood
|
|
Finnal update. I estimate that I got around .2 ml of oil. That may seem like a little but it is a sucsess for me, by proving the setup works for oil
extaction.
I am going to get a large flask and start growing lots of fragrant plants now.
|
|
|
happycamper723
Harmless

Posts: 10
Registered: 23-4-2012
Member Is Offline
Mood: Curious
|
|
This actually happens to be my area of expertise...
What oil are you thinking about distilling, specifically? Or are you just trying to generally make an extract? Or a hydrosol?
Various oils have various distillation techniques. Many oils require special distillations because they are damaged by standard distillation.
Geranium oils are easy enough--but look out. Steam distillation is your only option and a lot of the oils in Geranium have a relatively high boiling
point that may also damage them. For instance, Citronellol has a boiling point of 225 degrees Centigrade. Some of the more minute oils and more
specifically, terpenes and terpenoids, have an even higher boiling point.
I hope your thermometer reaches at least 300 degrees centigrade, because you'll need to get to about 250 to guarantee all of the oils have been boiled
off.
P.S.
Love your Youtube channelIt helps me out a ton
[Edited on 6-6-2012 by happycamper723]
[Edited on 6-6-2012 by happycamper723]
"Science, eh? Hmmm...I think I read somewhere that science is used to make BOMBS! TERRORIST!!!"
-Homer Simpson
|
|
|
99chemicals
Hazard to Others
  
Posts: 174
Registered: 24-3-2012
Location: In the Octet
Member Is Offline
Mood: No Mood
|
|
Eventualy I will want to do various mints like pepper and spearamint maybe lemon(I don't know you you would do that. Zest? Peel? Whole lemon?). Do
you know if the setup used above will work?
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
If you have some I highly suggest extracting the hydrosol(the cloudy water) with Dichloromethane due to its low boiling point it will allow u to
cleanly separate the DCM.
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
White Yeti
National Hazard
   
Posts: 816
Registered: 20-7-2011
Location: Asperger's spectrum
Member Is Offline
Mood: delocalized
|
|
This is a nice set-up, and a success indeed. The key with extracting oils from plants is an issue we often ignore, scaleability. You could scale up
glassware, but it would be fantastically expensive. My summer project is going to be the steaming of plant matter in oil drums. I've got 4 oil drums,
at least 3L each. I plan on heating everything atop some sort of wood stove and condensing the vapours with polyethylene tubes immersed in cold water.
I always thought that the oils would interact with the polyethylene, but seeing how little comes over, I think this isn't going to be a major issue.
"Ja, Kalzium, das ist alles!" -Otto Loewi
|
|
|
Farmerjohn
Harmless

Posts: 8
Registered: 14-6-2012
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by White Yeti  | This is a nice set-up, and a success indeed. The key with extracting oils from plants is an issue we often ignore, scaleability. You could scale up
glassware, but it would be fantastically expensive. My summer project is going to be the steaming of plant matter in oil drums. I've got 4 oil drums,
at least 3L each. I plan on heating everything atop some sort of wood stove and condensing the vapours with polyethylene tubes immersed in cold water.
I always thought that the oils would interact with the polyethylene, but seeing how little comes over, I think this isn't going to be a major issue.
|
I wonder how hard and feasible would it be to take some scrap water pipe {I know the salvage yard has large 3-4 in copper pipes} and with some copper
tubing make a scaled up soxhlet extractor and condenser with common plumbing pipe and adaptors?
|
|
|
White Yeti
National Hazard
   
Posts: 816
Registered: 20-7-2011
Location: Asperger's spectrum
Member Is Offline
Mood: delocalized
|
|
In the case of essential oil extraction, a soxhlet extractor is not ideal. What you're looking for is a way to evaporate off the oils without
destroying them, so a once through design. A soxhlet extractor would almost certainly denature the oils after a few cycles. Besides, water is
plentiful. The design of a soxhlet extractor is focused on the conservation of solvent.
"Ja, Kalzium, das ist alles!" -Otto Loewi
|
|
|