| Pages:
1
2 |
ScienceNoob
Harmless

Posts: 19
Registered: 3-6-2012
Member Is Offline
Mood: No Mood
|
|
Need Help with Fractional Distillation of Camphor oil
I am currently distilling a oil which is currently boiling at 170C. Here is the problem I have been running this distillation for over 13 hours the
abstract says that it should only take 4 hours. I recently ran a test run with tap water using my water aspirator to create a vaccum. I found it odd
that even with the vaccum attached that the BP was 100C this seems odd because this is what it should boil at at normal pressure please correct me if
I'm wrong. I was expecting my thermometer to get a reading by now and to have my distillate come over to the receiving flask by now. I will post
pictures if someone gets n here to help.
|
|
|
Hexavalent
International Hazard
    
Posts: 1564
Registered: 29-12-2011
Location: Wales, UK
Member Is Offline
Mood: Pericyclic
|
|
The BP of water will vary with atmospheric pressure - could be 97*C in some places, maybe even 102*C in others.
Silly question, but are you certain the vacuum hose is actually attached to the apparatus?
Are you using a vacuum trap between the flask and pump/aspirator? What sort of water pressure is running through the aspirator?
Post the pictures and add more detail about your situation and we'll try and help.
[Edited on 3-6-2012 by Hexavalent]
"Success is going from failure to failure without loss of enthusiasm." Winston Churchill
|
|
|
ScienceNoob
Harmless

Posts: 19
Registered: 3-6-2012
Member Is Offline
Mood: No Mood
|
|
TY TY TY stay there please I will post pictures right now to show you what i'm working with just hang tight please I beg you
|
|
|
Hexavalent
International Hazard
    
Posts: 1564
Registered: 29-12-2011
Location: Wales, UK
Member Is Offline
Mood: Pericyclic
|
|
I will, as long as you discontinue your practice of asking in other, unrelated threads for help with this question.
This should be in Beginnings, I'll inform the mods so that they can shift it.
"Success is going from failure to failure without loss of enthusiasm." Winston Churchill
|
|
|
ScienceNoob
Harmless

Posts: 19
Registered: 3-6-2012
Member Is Offline
Mood: No Mood
|
|
I apologize I'm just desperate 
|
|
|
Hexavalent
International Hazard
    
Posts: 1564
Registered: 29-12-2011
Location: Wales, UK
Member Is Offline
Mood: Pericyclic
|
|
Patience is key to chemistry. If I were you, I'd switch everything off now and try again tomorrow or later, depending on where you are in the world
(time zone areas), e.g. in the UK ATM it's 22:20 and getting quite late. Concentration will rapidly decrease and you will likely, under this
additional stress of yours, break something or hurt yourself; trust me, my 2L quickfit flask full of sodium hypochlorite knew this.
"Success is going from failure to failure without loss of enthusiasm." Winston Churchill
|
|
|
ScienceNoob
Harmless

Posts: 19
Registered: 3-6-2012
Member Is Offline
Mood: No Mood
|
|
Well i can't find my flash drive atm but yes I am sure the hoses are connected properly the condenser is filling nicely and the outlet is making a
small little tornado where the water goes back into the tub please wait just a little longer I will have photos soon.
|
|
|
Hexavalent
International Hazard
    
Posts: 1564
Registered: 29-12-2011
Location: Wales, UK
Member Is Offline
Mood: Pericyclic
|
|
How much oil are you distilling and how long is the fractionating column you are using?
Little amount of oil and large column = no brainer.
How do you know BTW it's boiling at 170*C? Did you look this value up or measure it yourself, e.g. with an infrared thermometer or something?
[Edited on 3-6-2012 by Hexavalent]
"Success is going from failure to failure without loss of enthusiasm." Winston Churchill
|
|
|
ScienceNoob
Harmless

Posts: 19
Registered: 3-6-2012
Member Is Offline
Mood: No Mood
|
|
I'm fine ready to go it's only 5:00 pm where I am at plus I have insomnia  posting some pictures now
posting some pictures now

      
|
|
|
Hexavalent
International Hazard
    
Posts: 1564
Registered: 29-12-2011
Location: Wales, UK
Member Is Offline
Mood: Pericyclic
|
|
How are you actually heating the oil?
I can't see in the photos.
"Success is going from failure to failure without loss of enthusiasm." Winston Churchill
|
|
|
ScienceNoob
Harmless

Posts: 19
Registered: 3-6-2012
Member Is Offline
Mood: No Mood
|
|
Yes I have an infra red thermometer but I know that the real BP is when it reaches the 3 way adapter which leads me to believe something is wrong with
the aspirator.. I got it to work before with a 500mm vigerux column but I broke it being a noob trying to unpack with a small coat hanger. Feel free
to call me dumb because I smacked myself literally could of just used muratic acid. Also I am distilling roughly 200 ML of the Oil at the moment. Do
you think using a larger flask would be better I have a 1000ML RBFlask as well as a 3 neck RBFlask. I don't think its the flask but I'm just reaching
out at any straw possible at this point
|
|
|
ScienceNoob
Harmless

Posts: 19
Registered: 3-6-2012
Member Is Offline
Mood: No Mood
|
|
yes I am using an oil bath peanut oil since it retains heat nicely and doesn't catch on fire from what I have read until it reaches above 200C
 
|
|
|
ScienceNoob
Harmless

Posts: 19
Registered: 3-6-2012
Member Is Offline
Mood: No Mood
|
|
Also to answer your other question I am using a 200MM vigerux for my column
|
|
|
Hexavalent
International Hazard
    
Posts: 1564
Registered: 29-12-2011
Location: Wales, UK
Member Is Offline
Mood: Pericyclic
|
|
I would use a 500ml flask, if you have one.
Too big a flask will cause problems with heating, splashing and maybe bumping...always use some boiling stones or a stir bar.
Too small a flask will cause problems with filling the excess headroom with vapours.
1.. If it's reaching the 3-way adapter, what's the problem?
3. Do you have a vacuum gauge? If so, measure the vacuum produced and compare it with the expected boiling point at THAT negative pressure level.
4. Does the aspirator have fluctuations in the vacuum it provides?
5. What flowrate of water is going through said aspirator?
6. Add some marshmallows to a sealed vacuum flask and quickly apply a vacuum and release it. Does anything happen to them?
7. Is the vacuum tubing collapsing maybe under the vacuum?
Also, please use the edit feature to add more information to a single post as opposed to posting sequentially.
Edited to remove questions answered whilst I was typing this post.
[Edited on 3-6-2012 by Hexavalent]
"Success is going from failure to failure without loss of enthusiasm." Winston Churchill
|
|
|
ScienceNoob
Harmless

Posts: 19
Registered: 3-6-2012
Member Is Offline
Mood: No Mood
|
|
I am using a 250RB Flask for the reaction vessel.
1. How are you heating the oil? Peanut Oil Using Heating plate with magnetic stirring
2. If it's reaching the 3-way adapter, what's the problem? It is not reaching the 3 way adapter
3. Do you have a vacuum gauge? If so, measure the vacuum produced and compare it with the expected boiling point at THAT negative pressure level. Nope
I don't have one need to get one
4. Does the aspirator have fluctuations in the vacuum it provides? No this is a pond pump just drop it in the water and it does its thing
5. What flowrate of water is going through said aspirator? It seems to give a pretty good flow rate I can't really give a measurement because I'm not
sure how to test that.
6. Add some marshmallows to a sealed vacuum flask and quickly apply a vacuum and release it. Does anything happen to them? I will have to try this
7. Is the vacuum tubing collapsing maybe under the vacuum?
|
|
|
Hexavalent
International Hazard
    
Posts: 1564
Registered: 29-12-2011
Location: Wales, UK
Member Is Offline
Mood: Pericyclic
|
|
1. I would suggest using a bigger flask.
2. My mistake, sorry I misread one of your posts. Have you tried warming the oustide of the column with a hot air gun or something? It looks like you
are performing this outside, and if its a windy day even with insulation the column can cool quickly. Can you see a reflux effect of the boiling oil
on the base of the fractionating column?
4. Are you using a water aspirator for the vacuum? How does a pond pump create a vacuum?
5. High pressure? Low pressure? Trickle?
6. Just to test the vacuum.
7. Collapsed tubing caused by thin-walled hose is a bad thing for containing a vacuum. Shoot us an image of the aspirator/vacuum pump if you can, in
operation, with the tubing you use. What is the wall thickness of this tubing?
"Success is going from failure to failure without loss of enthusiasm." Winston Churchill
|
|
|
ScienceNoob
Harmless

Posts: 19
Registered: 3-6-2012
Member Is Offline
Mood: No Mood
|
|
Ok I will post pics here in a minuet.. I think it may be the tubing here is the link http://www.hometrainingtools.com/rubber-tubing-5-16-x-10/p/C...
The pressure is I would say low pressure like turning a faucet on really low... and II am now sure how the pond pump creates vaccum I was just going
by what the procedure recommended. Also, I can see the reflux swirling around mixing with larger pools of precipitate then they get heavy and fall
back in the reaction vessel. The precipitate is all the way up to the male piece of the 3 way adapter I think I am going to use a blow dryer to see if
that helps it. Posting pics in 5 mins as requested
|
|
|
Hexavalent
International Hazard
    
Posts: 1564
Registered: 29-12-2011
Location: Wales, UK
Member Is Offline
Mood: Pericyclic
|
|
It states on the HST website 'but is too flexible for vacuum application' . . .and this is what you are using it for.
What do you mean 'the precipitate'? Do you know what a precipitate is?
"Success is going from failure to failure without loss of enthusiasm." Winston Churchill
|
|
|
ScienceNoob
Harmless

Posts: 19
Registered: 3-6-2012
Member Is Offline
Mood: No Mood
|
|
Sorry wrong word thus the name ScienceNoob umm hows about condensation or to use non science vocab I can see liquid speckles working up into the
adapter. Yes that is what I'm using I didn't know any better I know learn to read is the answer I was just excited when I was ordering everything and
made quite a few mistakes. However funny thing is I got this to work before using the same tubing just a different column was about the only thing
different. I changed out the tubing real quick to new tubing which hasn't been used yet or exposed to any natural elements such as the sun. I have a
question though I am using the infrared temp gun to see what the temp is up the column it is about 23 C outside the three way adapter is about 38.5C
is this a good sign that it may be ready to come over soon?
|
|
|
Hexavalent
International Hazard
    
Posts: 1564
Registered: 29-12-2011
Location: Wales, UK
Member Is Offline
Mood: Pericyclic
|
|
That is called the condensation front, and when it has tricked into the condenser it is called the fraction, which is the distillate.
No, the outside temperature of the 3-way adapter and the BP of the liquid at 170*C suggests that the liquid will reflux and drip back into the flask
WAY before going anywhere near the condenser.
Is it feasible to do the distillation indoors where there is no wind? Add some fibreglass wool insulation between the layers of foil if possible and
if necessary heat the outside gently with a heat gun or hairdryer. Turn off the magnetic stirrer for about 10 seconds... .when the vortex has
disappeared, can you see bubbles forming and rising to the top? This will probably tell you if it's boiling or not. Be certain when measuring with
the infrared gun to hit the temperature of the liquid, not the hotplate itself. Can you see any other signs of actual boiling? Can you see little
drops of liquid entering the flask again from the bottom of the fractionating column?
Also, how fast is the water entering the aspirator? A low speed (low pressure) will produce a very weak vacuum . . .perhaps your mains water pressure
was different on that day where it worked.
Is this, or something similar, what you have for supplying the vacuum?

[Edited on 3-6-2012 by Hexavalent]
"Success is going from failure to failure without loss of enthusiasm." Winston Churchill
|
|
|
ScienceNoob
Harmless

Posts: 19
Registered: 3-6-2012
Member Is Offline
Mood: No Mood
|
|
here is the aspirator and tubing picture

|
|
|
Hexavalent
International Hazard
    
Posts: 1564
Registered: 29-12-2011
Location: Wales, UK
Member Is Offline
Mood: Pericyclic
|
|
Can you post some pictures;
a) With the entire setup in view
b) With the flask in view, WITHOUT magnetic stirring being switched on
c) With the entire aspirator setup in view??
"Success is going from failure to failure without loss of enthusiasm." Winston Churchill
|
|
|
ScienceNoob
Harmless

Posts: 19
Registered: 3-6-2012
Member Is Offline
Mood: No Mood
|
|
Yes I have seen it Reflux (I am pretty sure this is when the condensation? drips back down into the reaction vessel) quite a few times I have not
tried to turn of the magnetic stirrer yet I will try that here in a few minuets. I can't do it inside no one trusts me really I don't trust myself yet
either. I did pack it the inside with some steel wool I thought this would help with the heat problem. I also thank you for the clarification on
vocabulary very foolish to use words that you are unsure of the definition of said word or words. I also see no little drops of liquid at the bottom
of the column it is all trying to work its way up at the moment. The adapter is getting very foggy though but the thermometer just won't jump.
|
|
|
ScienceNoob
Harmless

Posts: 19
Registered: 3-6-2012
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Hexavalent  | That is called the condensation front, and when it has tricked into the condenser it is called the fraction, which is the distillate.
No, the outside temperature of the 3-way adapter and the BP of the liquid at 170*C suggests that the liquid will reflux and drip back into the flask
WAY before going anywhere near the condenser.
Is it feasible to do the distillation indoors where there is no wind? Add some fibreglass wool insulation between the layers of foil if possible and
if necessary heat the outside gently with a heat gun or hairdryer. Turn off the magnetic stirrer for about 10 seconds... .when the vortex has
disappeared, can you see bubbles forming and rising to the top? This will probably tell you if it's boiling or not. Be certain when measuring with
the infrared gun to hit the temperature of the liquid, not the hotplate itself. Can you see any other signs of actual boiling? Can you see little
drops of liquid entering the flask again from the bottom of the fractionating column?
Also, how fast is the water entering the aspirator? A low speed (low pressure) will produce a very weak vacuum . . .perhaps your mains water pressure
was different on that day where it worked.
Is this, or something similar, what you have for supplying the vacuum?

Nope what you see in the picture is the so called aspirator aka Pond Pump attached to an adapter which is attached to the water hose inlet which is
attached to the condenser when I turn on the pump it fills really quick like within maybe 20 seconds? I will go back down now to get better pictures
my lab top is not ideal for this lol
[Edited on 3-6-2012 by Hexavalent] |
|
|
|
Hexavalent
International Hazard
    
Posts: 1564
Registered: 29-12-2011
Location: Wales, UK
Member Is Offline
Mood: Pericyclic
|
|
May I ask, Mr Noob, how old you are?
I can also see from your earlier photos that your thermometer is over-immersed, which can give an inaccurate reading. The base should be parallel with
the side arm;
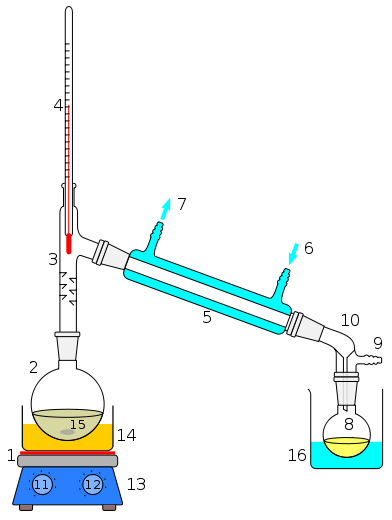
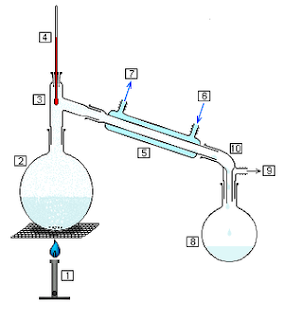
[Edited on 3-6-2012 by Hexavalent]
"Success is going from failure to failure without loss of enthusiasm." Winston Churchill
|
|
|
| Pages:
1
2 |