| Pages:
1
2 |
BromicAcid
International Hazard
    
Posts: 3245
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Making Lithium Metal
I found it odd that in my quest to find a reliable procedure for the procurement of sodium metal from its commonly available salts I would stumble
more often then not over detailed procedures for the production of lithium in the lab setting instead. Now, not wanting to be wasteful in my hording
of information I present the culmination of the methods that I have run across. People are welcome to elaborate on any given technique, criticize its
effectiveness, or even add their own, this is the lithium production thread, but hopefully I will be comprehensive enough during this initial stage as
to prevent it from growing to the size of the sodium thread.
Molten Salt Electrolysis
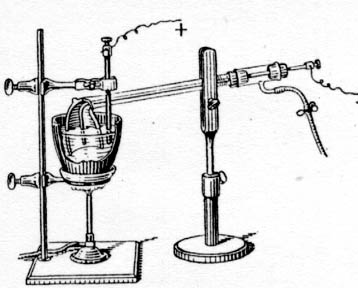
(From Complete Treatise on Inorganic Chemistry)
| Quote: | | In R. Bunsen's apparatus, an iron wire and graphite rod dip into the fused chloride without the aid of the clay pipe. In a few seconds, says
Bunsen, a small silver-white regulus is formed under the fused chloride round the iron wire, and adhering to it, which, after 2 or 3 minutes, attains
the size of a small pea. To obtain the metal, the wire pole and regulus are lifted out of the fused mass by a small, flat, spoon-shaped iron spatula.
The wire can then be withdrawn from the still molten metal, which is protected from ignition by the lithium chloride with which it is still coated.
The metal, after cooling under rock oil, may now be easily taken off the spatula with a pen-knife. As this operation can be repeated every three
minutes, an ounce of lithium chloride can be reduced in a very short time. In F. Hiller's modification, lithium chloride, mixed with ammonium
chloride, is heated to its m.p. in a porcelain crucible, See above figure. A piece of iron wire, passing though the bore of a clay tobacco pipe
inverted in the molten chloride serves as the cathode; and a rod of retort carbon serves as anode. For the sake of clearness, the drawing, has been
made as if the parts of the apparatus were transparent. If desired a stream of dry hydrogen gas can be passed into the tobacco pipe so that the
liquid about the cathode is not exposed to oxygen. A current from a battery giving 7 to 8 volts serves for the electrolysis of the molten chloride.
The reduced lithium rises to the surface of the liquid in the compartment where it is protected from oxidation. Before using the apparatus it is
advisable to cover the crucible and pipe in a layer of powdered graphite made into a slip with a dilute solution of lithium chloride. The coating is
dried and baked at red heat. This prevents the formation of lithium silicide by the action of the fused chloride on the clay. After an hours
electrolysis, the apparatus can be cooled, and the regulus of lithium can be removed by breaking the pipe. |
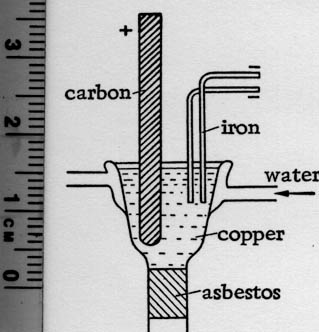
(From Preparative Inorganic Chemistry)
| Quote: | Electrolytic Preparation of Lithium
LiBr ---> Li + 1/2 Br
Pure Li is prepared (via the method of Ruff and Johannsen) from LiBr which is melted in an electric arc in the presence of 10 - 15% LiCl (the LiBr is
obtained from Li2CO3 by evaporating the latter from hydrobromic acid). The figure is a scale drawing (1:5 [Hence my inclusion of a ruler]) of the
Muthmann electrolysis vessel used for the melting procedure. It is made of copper and its upper part is cooled with water. While the melting point
of pure LiBr is about 546C and that of LiCl is 606C, a mixture of LiBr with 15% LiCl solidifies at 520C. The electrolysis proceeds at 10v. (as
measured across the terminals) and 100 amp. A graphite rod is used as an anode and two 4-mm. iron wires serve as cathodes. The metal which deposits
at the cathodes, is scooped up from time to time with a flat iron spoon and, while still liquid, is separated from the solidified melt on a cold stone
plate. It is next freed of adhering salt using Brochers' method, i.e., by immersion in a paraffin bath (180 - 200C). The salt settles to the
bottom, while the metal rises to the surface. After cooling, it is washed with ligroin. It is stored under ligroin (d. 0.56) in completely filled,
tightly closed vessels. |
Different Melts for electrolysis:
LiCl 52.9 mol% Li2CO3 19.8 mol% Li2SO4 27.2 mol% MP 445C Yield not stated
LiCl 75 mol % Li2CO3 25 mol % MP 507C Yield not stated
LiCl 50 mol % KCl 50 mol % MP 404C Yield not stated (Makes technical grade Li) | Quote: | From Comprehensive Inorganic Chemistry
Fused LiCl and KCl is electrolyzed in a cell containing a graphite anode and a mild steel cathode. The electrolyte is maintained at 404C, and the
voltage at 5.2, and the current density at 3.53 amp/sq in. |
LiBr w/ 10 - 15% LiCl MP 520C Yield 80%
Electrolysis in Non-Aqueous Mediums
From Complete Treatise on Inorganic Chemistry:
| Quote: | Lithium cannot be obtained by the electrolysis of aq. soln. of its salts, but L. Kahlenberg obtained it by the electrolysis of soln. of the chloride
(Reference 1) in pyridine, acetone, or in various alcohols. Silvery white lithium obtained from a conc. soln. of lithium chloride in pyridine at the
room temp. using a graphite plate as anode, and an iron plate as cathode with 0.2 to 0.3 amp. per 100 sq. cm. of cathode surface, and a potential
difference of 14 volts.
(Reference 1) S. von Laszczynsky, Organische Flussigkeiten als Losungsmittel fur anorganische Saltz, Berlin, 1894 ; Ber., 27. 2285, 1894 ; Zeit.
Elektrochem., 2. 55, 1895 ; S. von Laszczynsky and St. von Gorsky, ib., 4. 290, 1898 ; L. Kahlenberg, Journ. Phys. Chem., 3. 601, 1899 ; H. C. Patten
and W. R. Mott, ib., 8. 153, 1904 |
From Comprehensive Inorganic Chemistry:
| Quote: | | For example, the alkali chloride or bromide is fused with aluminum chloride or bromide and the fused mass is dissolved in nitrobenzene and
electrolyzed. By this method, lithium, sodium, potassium, and rubidium can be deposited on the cathode. The alkali ion serves as the cation, and the
aluminum appears in the complex anion. |
However, to my knowledge alkali metals produced in this way are impure. Also explosions can result and the yields are low. Additionally Li can be
produced from a solution of LiCl in liquid ammonia or hydrazine.
Purely Chemical Methods
Reduction of LiOH or Li2CO3 or LiCl with magnesium as stated in Complete Treatise on Inorganic Chemistry is explosive but 40% magnesium oxide can be
added to moderate the reaction. Information relating to chemical reduction by Mg or Al can be found if you see Warren, Chem. News, 1896, 74, 6, and
U.S.P. 2028390. Lithium salts such as LiNO3, Li2CO3, and LiOH can be reduced with carbon or metal carbides or even calcium at high temperatures.
Zirconium is an effective reducing agent for nearly any alkali metal that is combined with oxygen, it will reduce sulfates and phosphates in a self
sustained reaction.
From Comprehensive Inorganic Chemistry:
| Quote: | | For example, iron displaces the alkali metal sulfates and arsenates at their melting point, from thiocyanates at 650C, from borates and phosphates at
1300-1400C.... Alkali metal compounds such as hexacyanoferrates(II), cyanides, and azides can be decomposed into the alkali metal by heating.
|
However the Complete Treatise on Inorganic Chemistry states the lithium cannot be prepared by the reduction of lithium compounds with iron.
Other interesting things dealing with Lithium
From Descriptive Inorganic Chemistry 3rd edition, Geoff Rayner-Canham; Tina Overton:
| Quote: | | Liquid lithium is the most corrosive material known. For example, if a sample of lithium is melted in a glass container, it reacts spontaneously with
the glass to produce a hole in the container, the reaction being accompanied by the emission of an intense, greenish white light.
|
Lithium vapor is red!
Finally, an overview of the physical properties of lithium taken from here.
Atomic number 3
Atomic mass 6.941 g.mol -1
Electronegativity according to Pauling 1.0
Density 0.53 g.cm -3 at 20 °C
Melting point 180.5 °C
Boiling point 1342 °C
Vanderwaals radius 1.55 nm
Ionic radius 0.06 nm
Isotopes 2
Electronic shell 1s22s1 or [He] 2s1
Energy of first ionisation 520.1 kJ.mol -1
Standard potential - 3.02 V
Discovered Johann Arfvedson 1817
Note, lithium compounds are neuro toxins.
|
|
|
blip
Hazard to Others
  
Posts: 133
Registered: 16-3-2003
Member Is Offline
Mood: absorbed
|
|
Just a few days ago I extracted a few slivers of Li from Energizer e<sup>2</sup> Li batteries that I got for $18. Really I only started
extraction on one of the 8 batteries in the pack, supposedly giving me .94 g Li for $2.25 though I only have one reference to the supposed amount. At
another store the day before I began, I got great deals on butane lighters and mineral oil, the latter which without I could not store the Li or even
begin to halt any highly energetic reactions inside the open battery. I read up on <a href="http://www.rhodium.ws/">Rhodium's
site</a> about it much before and felt that I have no other easy source of an alkali metal, especially one that does not easily form peroxides
or superoxides. I strongly encourage anyone attempting this to try to do it all under mineral oil or whatever you plan to use, because what I think
is MnO<sub>2</sub> can be squeezed into more contact with the Li causing a reaction that could go out of control if you simply try to keep
the open segment "wet". The worst that has happenned under the oil bath was a red glolwing spark that went out, though prompting me to stop
for the day. In the air remaining "wet", twice it smoked with the first occurence glowing and that's when I dunked it into a container
full of oil for that purpose. Apparently, there was a piece of metal that I tugged on that initiated the reaction and only came out
"safely" completely under the oil.
I first removed the label, then successively cut near the anode two lines ~45° apart so that I could use pliers to peel. I monitored the
temperature and paid special attention to make sure that I didn't cut past the metal, which seems almost impossible. This killed the blade  so don't use your best one; perhaps you may have another method that is superior.
I freaked multiple times when when the battery warmed--due to me body heat and tight grip, combined with my tension! You may find a strip, one I
found was apparently Li but according to a reference there is another that isn't Li. Additionally, there are layers of Li and
MnO<sub>2</sub> in the large plastic roll. I used my pocket knife's scissors to cut through each layer of plastic after I had got
the Li on it. I do not know if one could cut open the entire battery and unroll the plastic film and then get a large layer of Li, but I may try it
when I next need Li for something (no use yet, so I expect it to be a LONG while considering my fright). Oh yeah, get used to all of the black
(supposedly red-brown) nitride that'll be all around when you're done. so don't use your best one; perhaps you may have another method that is superior.
I freaked multiple times when when the battery warmed--due to me body heat and tight grip, combined with my tension! You may find a strip, one I
found was apparently Li but according to a reference there is another that isn't Li. Additionally, there are layers of Li and
MnO<sub>2</sub> in the large plastic roll. I used my pocket knife's scissors to cut through each layer of plastic after I had got
the Li on it. I do not know if one could cut open the entire battery and unroll the plastic film and then get a large layer of Li, but I may try it
when I next need Li for something (no use yet, so I expect it to be a LONG while considering my fright). Oh yeah, get used to all of the black
(supposedly red-brown) nitride that'll be all around when you're done.
|
|
|
BromicAcid
International Hazard
    
Posts: 3245
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
For me I got lithium from taking apart military grade batteries from eBay. I got a Lithium/Sulfur dioxide battery for $2.99, it was about the size of
a lantern battery. I carefully took it apart and it was segmented into 8 smaller batteries.
So experimentally I drilled a hole into one of them and lo and behold like I wasn't expecting it, sulfur dioxide sprayed out as a mist. The
liquid bubbled out from the hole and the air was thick with it. So I took the battery and tossed it into some NaOH solution I quickly made up, the
SO2 was completely absorbed before bubbles broke the surface. After it stopped that I took a hack saw and cut around the battery and broke it in two.
It had a large wad in the middle of two different materials in rolls wound around one another.
Pulling the two of them apart I found that one was blackened and the other gray. I took a piece of the one and tossed it in water with no reaction.
The other though gave a definite indication that it was lithium, skittering around the surface and bubbling furiously. I quickly weighed the lithium,
5 g and balled it up and put it in mineral oil, after dunking it up and down several times it took on enough oil to make it more or less static in its
movement and pushed it to the bottom. And that is my story of extracting lithium metal from batteries.
However I really have no use for it, still doesn't mean I don't want to stock pile it. Going for electrolysis of the chloride in a week or
so, using a 1 1/2 inch copper endcap for pipe as my vessel. Bent some glass tubing around the top for cooling, easy to obtain the iron wire and the
graphite anode was also easily obtained.
|
|
|
Strepta
Harmless

Posts: 44
Registered: 6-5-2004
Member Is Offline
Mood: No Mood
|
|
Lithium from Pyridine
posted by BromicAcid
[Lithium cannot be obtained by the electrolysis of aq. soln. of its salts, but L. Kahlenberg obtained it by the electrolysis of soln. of the chloride
(Reference 1) in pyridine, acetone, or in various alcohols. Silvery white lithium obtained from a conc. soln. of lithium chloride in pyridine at the
room temp. using a graphite plate as anode, and an iron plate as cathode with 0.2 to 0.3 amp. per 100 sq. cm. of cathode surface, and a potential
difference of 14 volts. ]
Last December I thought I'd try the electrolysis of LiCl in pyridine, so I purchased 500ml of pyridine for $60 (ouch!!) I used a graphite anode
and a nickel cathode (approx. 2 sq. in.) Spacing between electrodes was about .75 in. I was able to dissolve 2 g of LiCl in 30 ml of pyridine.
Applying voltage, I could hardly detect any current flowing. For 30v across the electrodes there was perhaps 15ma of current. The LiCl must not be
ionized. Doing a little math after the fact, it takes about 100,000 coulombs to reduce a mole (7 grams) of Li+ ions to Li. At .015A that would take
about 2000 hours or much larger plates and I have better things to do. Also, pyridine is not all that pleasant to have around as it smells a lot like
skunk. Live and learn I guess.
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
J Phys Chem
I read his articles (there are later ones in that journal) and thought that there may be some cottage-industryness to it if there was enough demand
and cheap electricity. But if I remember correctly, it is a very touchy procedure, for several reasons. The pyridine must be very dry, and stay that
way. He did not get good results with the other solvents, either. Would take some work, and this would probably not be a side project, but something
you would devote a lot of effort towards, like Kahlenberg did.
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
How about DMF or DMSO?
DMSO is pretty readily available in the states I hear.
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
BromicAcid
International Hazard
    
Posts: 3245
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Currently warming my frozen bottle of DMSO up under my arm pit. If the weather holds I will get back to this in a bit. Burrrr...
The solubility of lithium compounds and analogous sodium compounds (but we all know how lithium compounds differ from the other alkali metals on some
things) have persuaded me to give this a shot. As well as the lack of reactivity of Na in DMSO until a high temperature and that DMSO is an ionizing
solvent. Too bad I don't think my DMSO is anhydrous, might have to dry over Na wire first.
Edit 1: Got too cold holding the DMSO in every crevasse of my body so I put it in a pan of hot water to heat up. Went out back and found out that I
was out of LiCl  so I had to neutralize some carbonate with HCl so this is going to
take awhile, stupid hygroscopic/disquecent LiCl, it's boiling away right now though. so I had to neutralize some carbonate with HCl so this is going to
take awhile, stupid hygroscopic/disquecent LiCl, it's boiling away right now though.
Edit 2: Gahhhh DMSO dripped on the side of the bottle or something and it got on my skin, stupid 99% DMSO, it leaves the most rank aftertaste I can
describe and it just enters your body where it pleases.... Grrrr...
Edit 3: LiCl dry and commencing electrolysis, notice my axehandle style editing today 
[Edited on 5/16/2004 by BromicAcid]
[Edited on 5/16/2004 by BromicAcid]
[Edited on 5/16/2004 by BromicAcid]
|
|
|
BromicAcid
International Hazard
    
Posts: 3245
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Very Interesting...
My write-up as play by play style:
20ml of DMSO was placed in a small test tube and 1 chunk of LiCl of approximately 1.2 g was added to the DMSO. The piece was crushed with the aid of
a stirring rod and a powder remained at the bottom of the test tube. It should be noted that my DMSO was 99% and was not subject to additional
purification and that my LiCl although freshly dried from solution probably still contained a percentage of H2O along with metal ions present in the
OTC HCl used in its preparation.
A nickel anode and cathode were placed into the solution and electrolysis was begun at 6V. Immediately bubbles began to form at the cathode and the
after a few moments the anode started to take on a blue hue that gradually began to settle to the bottom forming some nickel salt, NiCl2 probably.
A further minute or two passed and the cathode started to turn black and a red/brown/black cloud started to plume up from it to the surface. The
solution began to gradually darken but the red color still stuck largely to the top and continued to rise from the cathode as nickel salts continued
to descend and cover the very bottom of the test tube.
A very strange smell started to come from the solution, not unlike rotting eggs but entirely different from H2S. By this time the voltage had be
raised to 12V with the visual clue being the increase in evolution of bubbles and red liquid from the cathode. The red liquid had a distinct top
layer at this time but it could have just been the meniscus of the test tube refracting the color oddly.
After about 8 minutes from the commencement of electrolysis the application of electron movement to the solution was discontinued. Still LiCl
remained undissolved at the bottom and there was a color of Ni salt at the bottom in solution. The red color was at the top and the electrodes were
removed from the solution, I noticed by this time a somewhat intense garlic/onion smell.
The anode electrode was somewhat clean and shiny but slightly pitted. At the very end LiCl was stuck to it coated in nickel salts turning it blue.
The cathode was black with nasty looking flecks and chunks covering it. It had an unusual smell even different then the solution that escapes my
descriptive capabilities. As I inspected it, it started to fume in the air. My heart almost skipped a beat, I spit onto my work bench and rolled the
electrode in it (My surefire test for alkali metals, although not 100% reliable, the reagents are readily avalible  ), and it started sizzling and boiling on the surface, lithium metal had been achieved
as a non-adherent black coating. Holding the test tube up to the sun I noticed flecks throughout the solution, "Lithium?" I thought to
myself. ), and it started sizzling and boiling on the surface, lithium metal had been achieved
as a non-adherent black coating. Holding the test tube up to the sun I noticed flecks throughout the solution, "Lithium?" I thought to
myself.
I added a bit of H2O to the test tube, only a tiny bit and the darkness immediately began to dissipate in a disproportionate manner to the water I
added, much more so then could be accounted for by dilution. A few of the black shavings in the solution bubbled away but most stayed suspended
unaffected.
I rinsed out the testube with water and got even more weird smells. The anode washed clean instantly but the cathode had an adherent coating on most
of it that would hardly scrape off, NiO?
Regardless, it was an interesting experiment, thank you Vulture for your idea! My guess is that the lithium being generated at the cathode was just
reducing the DMSO. In the end the solution was luke warm, to be expected from the heat of electrolysis. Lots of pretty colors though. 
Edit: Looked up the Rxn. between alkali metals and DMSO, supposedly sodium and potassium react with DMSO by cleaving the carbon-sulfur bond:
CH3SOCH3 + 2Na ---> CH3SONa + CH3Na
CH3SOCH3 + CH3Na ---> CH3SOCH2Na + CH4
"Electrolytic reduction of NaCl or NaI in DMSO similarly leads to a mixture of hydrogen and methane gasses at the anode." Funny thing is I
only got gas evolution at the cathode....
[Edited on 5/16/2004 by BromicAcid]
|
|
|
BromicAcid
International Hazard
    
Posts: 3245
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
I've been trying to figure out all the reactions that were occuring during my electrolysis of LiCl in DMSO. My anode reaction seems
straightforeward:
Ni(s) ---> Ni2+ + 2e-
The charge being balanced by the chlorine anions still in solution.
However my cathode reaction is a bit more complex. I would like to think that the only thing occuring would be:
Li+ + e- ---> Li(s)
However with all the colors and bubbling that occured there was a bit more to it.
Sure, the lithium may have depositied and almost instantly reacted away but what was the end product.
My cathode was covered in a black adherent coating, NiS, NiO, C ? I need to do more tests. The bubbles generated could have been CH4 as supplied by
my above set of equations:
CH3SOCH3 + 2Li ---> CH3SOLi + CH3Li
CH3SOCH3 + CH3Li ---> CH3SOCH2Li + CH4
But what about those other products, CH3SOLi, CH3SOCH2Li that were formed, any one have any information on the color of these compounds possibly to
explain the red/black/brown plume rising from the cathode maybe the lithium reduced the DMSO to DMS? And how about methyl lithium, supposedly it is a
somewhat common organic reagent and somewhat stable, how fast would anyone here guestimate the rxn between the methyl lithium and DMSO would be in
terms of a rate?
Really, I must try this experiment again, it was very interesting to say the least. Numerous color changes, lithium metal, many lithium-organic
compounds in solution. How about any other ideas as to the identity of the gas produced at my cathode? The information that I have on DMSO reacting
in this manner with NaCl states that gas evolution should come from the anode, however logic would dictate that the lithium would be produced at the
cathode and react with the DMSO there, why would it have to react at the anode, that part is a chemical reaction, not an electrochemical reaction.
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
I'm not really sure but...
When the Ni becomes Ni2+, the Ni2+ replaces Li+ as the strongest oxidizing agent so it will be preferentially plated onto the cathode before lithium
metal. your black coating could just be a fine layer of nickel plating. Why did you choose a nickel anode and not graphite or another inert
electrode? I am not sure what the gas formed was.
This may be too simplistic an explanation or not aplicable.
[Edited on 18-5-2004 by rogue chemist]
|
|
|
BromicAcid
International Hazard
    
Posts: 3245
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
I know I did get some lithium but yes, I did neglect that the nickel could just be re-plating on the cathode. I used nickel because I bought a 2 Kg
roll of nickel wire awhile back and I made 100 .3m electrodes from it, so they are essentially stock piled. I will have to run this electrolysis with
an inert electrode. Still though, the colors in the solution are perplexing, it turned almost black but a ml or two of water turned it just to a
faint blue of nickel salts.
|
|
|
BromicAcid
International Hazard
    
Posts: 3245
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Today was my day of electrolysis of LiCl in non-aqueous mediums.
In DMSO: Tried this again with a graphite anode to prevent it from reacting. It greatly slowed the reaction from what I was getting with the nickel
anode, possibly the nickel cations were adding to the mobility of the electrons through the solution. But electrolysis did proceed, albeit slowly.
Also there was no color change at the cathode as there was before although gas evolution was noted as before. After quite some time I removed the
cathode and there was no noticeable deposit, it did bubble somewhat vigorously for a second when water was spritzed on it but whatever was there was
present in sub-gram amounts.
In Acetone: The reaction was almost nonexistent as judged by the amount of bubbles produced. Though there was a steady stream, it was nothing
considering I was throwing 12V 10A at it, solution finally obtained a very light haze to it but there was no cathode deposition. The electrodes were
very nearly touching in solution.
In Acetonitrile: Solution was nearly nonconductive, worst conductivity of the lot, electrodes were a hairs width apart and a bubble would emerge
every few seconds.
In Nitrobenzene: Second worst conductivity of the lot. Ran the reaction for a few minutes with almost no indication of reaction and my heart skipped
a beat when the electrodes touched under the solution and it sparked. I knew dinitrotoluene can explode even though it is highly oxygen deficient so
I had to worry a bit. No reaction at all in terms of a cathode deposit. (Also, I didn't know I could get a nitro headache just from the
occasional waft of nitrobenzene vapors, well, live and learn)
In Methanol: This one was just for fun. The reaction was incredibly fast, the bubbles were coming up like tossing a piece of Mg into HCl. The
solution took on a yellow color very fast and the solution clouded up. The solution was boiling and electrolysis was discontinued. A precipitate
settled to the bottom.
Additionally not relating to this thread I tried NaBr in DMSO. The cathode bubbled but the anode turned orange/yellow and a solid started to
continually flake off it. Overall DSMO actually gave the best results. And with the graphite anode the solution stayed clear although the cathode
did take on a black color as before. Perhaps I could take what I have learned and try a mixed solvent?
[Edited on 5/23/2004 by BromicAcid]
|
|
|
Proteios
Hazard to Others
  
Posts: 109
Registered: 7-3-2004
Member Is Offline
Mood: No Mood
|
|
maybe THF is a good choice.....
literature drying of THF is over Na/K alloy....so its gotta be stable to the group 1 metals.
Dunno what conc. of Li salts you can get into THF... but I know for certain, lots of the Cs salts are really quite soluble in THF..... if you feel
very brave.
|
|
|
BromicAcid
International Hazard
    
Posts: 3245
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
I've found references to 1 M LiCl solutions in THF so it is therefore somewhat soluble. If I had some THF I'd give this a shot.
| Quote: | | lots of the Cs salts are really quite soluble in THF..... if you feel very brave. |
Nothing brave about it, it's just that cesium salts are usually quite expensive 
|
|
|
Proteios
Hazard to Others
  
Posts: 109
Registered: 7-3-2004
Member Is Offline
Mood: No Mood
|
|
An interesting problem!
However I would still reckon that the cost of the Cs salt itself would be nominal compared with the cost of isolating the Cs. It would take a brave
man to make more than 5g of Cs.... probably need to seal it in a ampoule...... vac line to get rid of the solvent... do the whole electrolysis under
Ar or something to keep oxidation down..... tricky buisness!
Actually now that i come to think about it Cs melts well below the BP of THF..... just do the electrolysis in degased THF..... Ar atm. with the
cathode over a test tube (at 40 C or something)... and with luck the molten Cs will pour off into the test tube..... simple eh?
Incidentally Ive been in chemistry a long time, and have never come across, or thought about isolation of group 1 metal by electrolysis of non-aq.
solution... its a really neat idea 
|
|
|
Marvin
National Hazard
   
Posts: 995
Registered: 13-10-2002
Member Is Offline
Mood: No Mood
|
|
A slightly more conventional idea for Cs might involve heating its chloride with calcium in a decent vacuum. Condense it directly in the ampule.
|
|
|
Proteios
Hazard to Others
  
Posts: 109
Registered: 7-3-2004
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Marvin
A slightly more conventional idea for Cs might involve heating its chloride with calcium in a decent vacuum. Condense it directly in the ampule.
|
CsCl dont melt till 600 and Ca 800.... Cs boils 650... what kind of reactor do you know that holds a good vacuum at this sort of temperature??? Ca
and CsCl woundt react at RT.... dunno about 600 C though.
In terms of conditions..... 40C, and a mild DC current would seem more accessible conditions that 0.5 thousand C... esp. when dealing with a chem.
that is violently oxidised.
|
|
|
Marvin
National Hazard
   
Posts: 995
Registered: 13-10-2002
Member Is Offline
Mood: No Mood
|
|
I'm not convinced its boiling point has much relevence in a vacuum, but melt one of them and there will certainly be a reaction even if there
isnt in the solid state.
Stainless steel for preference, quartz tube, but if I was doing it for small amounts Id probably suspend a little metal crucible in a flask and heat
it electrically. Keeping the whole thing inside a bag of inert gas would be good idea, that way if the flask implodes there isnt a risk of explosion.
Reduction with calcium has a number of advantages, its quick, its pure and not least we know it works. RT organic solvent electrolysis would have
some uses if the electrode current density can be made high enough to produce at a reasonable rate, but this assumes it works at all.
"0.5 thousand C"
Can you make that sound any more impressive? Gee, thats... thats... One and half thousand degrees Rankine!
It is violently oxidised, such as by oxygen dissolving in the solvent and the oxidised products forming at the other electrode... I cant help but
wonder if there would be problems with something as simple as sodium or potassium being produced quantitivly.
|
|
|
BromicAcid
International Hazard
    
Posts: 3245
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Trying lithium chloride in DMSO again under more controlled conditions.
An excess of LiCl was added to 25 ml of DMSO and placed in a test tube. .05 g of Na metal was added to try to ensure the solvent to be somewhat
anhydrous, and although sodium reacts with DMSO on it's own, the reaction was very slow, leading me to believe water contamination was not
extensive.
A rubber stopper was put into the top of the test tube and two small holes were made to allow for two nickel electrodes to enter the solution.
Electrolysis was begun, 6V, 300 ma and has been going for 30 minutes as of now.
The cathode is covered with a black substance and bubbles continuously, as usual the anode is going into the solution and sinking straight to the
bottom. There is a very sharp division in the color of the electrolyte, the top layer, about 3/4 of it, is dark brown now, and the bottom 1/4 is light
blue with not a hint of brown, going to let this go another 8 hours or so and see what I end up with.
One of the products depositing or coloring the solution might be methyl lithium, anyone know a way I could test for this?
Edit #1: An hour has passed since this post. The solution no longer looks brown, it is now a very deep red through the top 3/4 of the test tube.
The blue at the bottom has yet to mix. There is lots of solid dispersed throughout the mixture now, dark red flakes. I saw the top of the mixture
and was ecstatic for a moment upon viewing the beautiful clear crystals that were appearing there, till I realized it was just my DMSO freezing. It
was carefully heated as to not disturb the two layers, and now I leave it for another hour.
Edit #2: It's been going for over 2.5 hours so I went out to check on it. The red flakes had settled out somewhat to the bottom of the red
layer and the blue layer remained separate. I decided that I should take some pictures, so I did. Then I decided that I wanted to stir it up, maybe
the lithium percentage in the solution had dwindled and the exchange with the bottom solution was too low. So I took out the stopper... wait... tried
to take out the stopper, the test tube broke when that happened. And guess what, I got a few ml's of that solution containing who knows what on
my hand. DMSO solutions of lithium ions and more are not nice. Pure DMSO actually works considerably less effectively at transporting ions across
the skin barrier, it works best in the 85 - 95% range when combined with water. So I dashed to my shed and rinsed with acetone, then water. I think
I'm starting to get a headache. Nevertheless, I got a new test tube, retransferred the solution, now a homogenous color and consistency to a new
test tube and recommenced electrolysis, I'm eager to see if it resettles. (Note, the electrodes once removed gave no indication of lithium, in
my previous runs there was evidence of it, possibly high currents are necessary to deposit it faster then it reacts with its surroundings.)
Edit #3: A little over 3.5 hours have passed. The solution has somewhat resettled. There appears to be a difference in density of the solution, the
solid has settled somewhere in the lower area of the test tube, but not at the bottom. There is no longer a distinction of colors evident, it's
all red, but I can see into the solution now that the solid has settled. I can taste DMSO, but my headache went away.
Edit #4: And thus it continues. Everything settled out. There was a thick layer of whatever the reaction is making on right above some of the solid
LiCl remaining in the test tube. The solution was dark red, but did not contain appreciable suspended material. It is still going. I shook the
reaction mixture and now it looks like mud. But it's already starting to settle, I think the precipitate is aging nicely and settles easily as a
side effect.
Edit #5: Total of 5 hours has passed. I removed the electrodes, no trace of lithium. Thinking that I may have somehow formed a lithium complex
imbedded in a sulfur crown I added some of the red liquid in some benzene to see if any of the color would transfer over, no. Added some of the
precipitate to water, the solution went clear but the precipitate made a suspension. The whole mixture heated up, not a lot, but it heated up
somewhat. That's it, done with DMSO for today.
[Edited on 10/25/2004 by BromicAcid]
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
Lithium batteries use a mixture of ethylcarbonate and dimethylcarbonate as elektrolyte for the Li<sup>+</sup> ions...
Dimethylcarbonate isn't very expensive, I could only find diethylcarbonate at acros.com though...
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
unionised
International Hazard
    
Posts: 5126
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
I think DMSO is doomed.
http://www.casper.organ.su.se/sop/sop401.html
|
|
|
BromicAcid
International Hazard
    
Posts: 3245
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Looking through a book yesterday titled "Industrial Electrochemistry" I found the lithium section to be fairly interesting and decided to reproduce it
here:
| Quote: | Lithium Electrowinning
Lithium is prepared by the electrolysis of fused lithium chloride in a lithium chloride - potassium chloride bath at 400 to 450C held in a cell
designed somewhat like a Dow magnesium cell or the Downs cell for sodium production so that the intermixing of the lithium metal and the chloride gas
is prevented (See Magnesium Electrowinning and Sodium, Electrolyte Production.) The LiCl-KCl mixture is approximately the eutectic mixture (about 45
per cent LiCl) whose melting point is 352C; the melting point of LiCl is 606C. Compared with the use of fused LiCl alone, this mixture improves
optimal efficiency, decreases corrosion problems, minimizes deteriation of the graphite anodes, and permits continuous operation.
The raw material is lithium chloride of high purity, particularly with respect to water and sodium content, although water can be removed to a degree
from the bath by pre-electrolysis at low amperage. Since lithium chloride is very hygroscopic, the handling of it to deter water pickup is a problem.
The physical properties of lithium and the LiCl-KCl eutectic mixture influence the cell design. Lithium melts at 179C and at 400C has a density of
0.49 g/cc, a viscosity of 0.402 centipoises, a vapor pressure much less then 1 mm Hg, and a surface tension of about 400 dynes/cm. The eutectic
mixture has a density of 1.65 g/cc at 450C and a viscosity of 5 centipoises at 500C. Lithium chloride has a decomposition potential of 3.684 v at
450C calculated from thermodynamic data, and the formal electrode potential for Li<sup>+1</sup> in eutectic LiCl-KCl at 450C is -3.41 v
measured against a Pt, Pt<sup>+2</sup> reference electrode. (See Fused Salt Electromotive Force Series.)
One type of cell used commercially is constructed of a covered steel pot suspended in a firebrick heating chamber, much like the magnesium cells. Gas
or oil is used to heat the cell. Top entering graphite anodes dip into the fused salt bath, and the steel cathodes are so proportioned that the
lithium rising from them to the surface of the bath is prevented from approaching the chlorine gas rising from the anode. Separate collecting means
are used for withdraw of the lithium and the chlorine. The molten lithium is carefully protected from contact with air, and is withdrawn from a
collecting vessel and cast into molds.
Another type of cell described recently<sup>1</sup> is designed like the Downs sodium cell. It is a covered, brick-lined cylindrical pot
26 in. in diameter and 30 in. deep with a single bottom entering anode 6 to 8 in. in diameter centered vertically in the pot. A steel cylindrical
cathode 9 to 12 in. inside diameter and 8 to 12 in. high surrounds part of the anode, and between them is a cylindrical diagram of perforated
24-gague, 316 stainless steel sheet, which keeps the chlorine rising along the anode from mixing with the lithium rising along the inside of the
cathode. A vertical hoodlike nickel chlorine collector is positioned over the top of the anode, and a steel collector placed over the cathode is used
to hold and guide the molten lithium to a vertical takeoff line continuously into a stainless steel holding tank. Auxiliary electric heating elements
in the bath maintain the temperature.
The lithium cells hold only a few hundred pounds of bath, and the production rate from cells using 900 to 1000 amperes is in the range of 8 to 10 lb
of lithium per day per cell.
Some typical operating data for commercial cells are given in table 1.
The cell described in Ref. 1 operates at somewhat lower voltage, current density, current efficiency, and unit energy consumption, and its chemical
efficiency is somewhat higher. While designed to operate at 1000 amperes, it can be operated up to 3500 amperes with no abnormal behavior.
Table 1. Lithium Cells-Operating Characteristics<sup>2</sup>
Current (amp) 850-900
Temperature
C 400-420
F 752-788
Voltage 8-9
Anodic c.d. (amp/sq. in.) 9.0
Cathodic c.d. (amp/sq. in.) 13.0
Current efficiency (%) 85-90
Unit energy (kwhr/lb) 18.2
Chemical consumption (LiCl/lb Li) 7.3
Chemical efficiency (%) 83.7
Cell capacity (lb) 220
Details about cell operation and about the physical properties and electrochemical behavior of lithium and its salts are presented in Refs. 1 and 2.
The separation of lithium isotopes 6 and 7 can be effected by the electrolysis in a mercury cathode cell and by electromigration techniques.
Information about both methods is given in the article. Isotopes-Electrochemical Separation; also see Electromigration in Liquid Metals.
References
1. Motock, G.T., Electrochem. Tech., 1, 122-127 (1963)
2. Landolt, P.E., and Sittig, M., chapter on "Lithium" in "Rare Metals Handbook," C.A. Hampel, Editor, New York, Reinhold Publishing Corp., 1961.
Clifford A. Hampel |
Makes designing and operating a cell to afford lithium metal from the chloride almost
sound reasonable eh?
|
|
|
Fleaker
International Hazard
    
Posts: 1252
Registered: 19-6-2005
Member Is Offline
Mood: nucleophilic
|
|
I have maybe 30 grams of lithium carbonate that I can dedicate to this reaction. I will use 300 mesh Li2CO3, 700 mesh Aluminum powder (in
stoichiometric ratios) and what I deem to be enough magnesium oxide to moderate the reaction rate. Up until that point, I'm not too worried, but my
problem is collection.
Since molten lithium is one of the most corrosive materials known, how should I contain the reaction? I was going to do it in a large evaporation dish
in a sand bath with a watch glass covering it. I was planning on filling the evap dish with dry ice/MeOH so that the lithium vapor would deposit on
the underside immediately (hopefully sparing the container). Eitherway, I'm worried about thermal shock. I hope the reaction is hot enough to vaporize
the lithium metal (which would facilitate separation) but not too hot to crack the porcelain and the watch glass. I think I might use a taller
reaction vessel or maybe use something with more thermal resistance than a flint watchglass. Any suggestions?
|
|
|
IrC
International Hazard
    
Posts: 2710
Registered: 7-3-2005
Location: Eureka
Member Is Offline
Mood: Discovering
|
|
Correct me if I am wrong but lithium will burn in sand, by pulling the oxygen right out of the silicon dioxide just like magnesium does. I think you
need an argon filled chamber. As far as I know burning lithium will even burn the oxygen out of glass. Fact is I don't even know how you would put
lithium out, it will suck anything oxygen or nitrogen (which makes a really explosive nitride) right from wherever it can get it. I have two bottles
each with 5 gm Li metal under oil I have never used as finding a safe way to do anything with it is hard. It makes me wonder why the chinese are so
cavalier with all the toys on the market using big Li ion cells. I have a radio controlled helicopter which loves to crash and I was waiting for the
day one of the cells starts burning. You should read the fire statistics in the U.S. for lithium batteries, mostly from improper charging using
something like nicad chargers instead of the proper source. Come to think of it lithium is fun stuff after all! Think of the fireworks we can play
with, of course blaming it all on a bad battery when the pyrotechnical police come looking for us.
|
|
|
neutrino
International Hazard
    
Posts: 1583
Registered: 20-8-2004
Location: USA
Member Is Offline
Mood: oscillating
|
|
Liquid lithium is one of the strongest reducing agents known, if not the strongest. I doubt it could be obtained by a simple thermite reaction,
although this one does involve distillation...
I doubt it will work, but it's worth a try.
Also, I think magnesium salts would just react with any lithium formed and liberate magnesium.
|
|
|
| Pages:
1
2 |
|