| Pages:
1
2
3 |
ParanoidAndroid
Harmless

Posts: 5
Registered: 8-12-2011
Member Is Offline
Mood: No Mood
|
|
What to do with tryptamine?
I have a large amount of pure tryptophan and recently came across an exceedingly simple method of making it into tryptamine: basically just reflux it
a mixture of xylene and acetone until CO2 stops evolving. A spearmint oil catalyst can be used to speed the reaction but adds a purification step
afterward and reduces yield.
My question is: what can I do with the resulting tryptamine? There are a whole slew of interesting substances that are related to tryptamine, but there's very little information on synthesizing those substances using tryptamine as a starting
material. Are there any easy ways to do this?
Of particular interest to me are AMT (an MAOI), serotonin (neurotransmitter), and DMT (which is an interesting precursor to other syntheses). The
molecular changes aren't complicated; there just isn't a lot of experimental info out there.
|
|
|
Bot0nist
International Hazard
    
Posts: 1559
Registered: 15-2-2011
Location: Right behind you.
Member Is Offline
Mood: Streching my cotyledons.
|
|
Decarboxylate and post a write up with the procedure, yield, and product identification ( melting point, solubility's, etc) before even worrying about
methylation.
[Edited on 22-12-2011 by Bot0nist]
U.T.F.S.E. and learn the joys of autodidacticism!
Don't judge each day only by the harvest you reap, but also by the seeds you sow.
|
|
|
Adas
National Hazard
   
Posts: 711
Registered: 21-9-2011
Location: Slovakia
Member Is Offline
Mood: Sensitive to shock and friction
|
|
Wow, if you really made it, it is a good news! You can make DMT (if it's legal in your country) or DIPrT - this is known to cause auditory
hallucinations only.
Rest In Pieces!
|
|
|
JibbyDee
Harmless

Posts: 38
Registered: 25-11-2011
Member Is Offline
Mood: No Mood
|
|
Very interesting. Have you successfully thermally decarboxylated tryptophan? Tryptamines fascinate me. I'm going to read TIHKAL one of these days, I
hear Shulgin did a serious amount of research on tryptamines.
[Edited on 23-12-2011 by JibbyDee]
|
|
|
zoombafu
Hazard to Others
  
Posts: 255
Registered: 21-11-2011
Location: U.S.
Member Is Offline
Mood: sciencey
|
|
PiHKAL and TiHKAL would probably be your best resources.
[Edited on 23-12-2011 by zoombafu]
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
Doubtful, he mostly obtains his typtamines using other starting materials or hard to get reagents. Google is your friend here.
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
turd
National Hazard
   
Posts: 800
Registered: 5-3-2006
Member Is Offline
Mood: No Mood
|
|
Actually Shulgin was eager to develop routes from uncontrollable materials and you will find that he published a route for most of his not ring
substituted tryptamines starting with tryptamine or at least gave an outline. Shulgin is a good, humble guy who lets his actions speak.
What he missed was the sodium triacetoxy borohydride (made in-situ) reductive methylation of tryptamine with formaldehyde to DMT. I have heard a few
success reports. Yields are low in an amateur setting, but given that it's only two steps from (dirt cheap) tryptophan, overall yield is better than
for most OTC routes to phenethylamines.
Original poster: Follow Bot0nist's advice and come back once you have geniune tryptamine. Some successful attempts have been posted on this forum.
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
I dont think alkylation that occurs via an imine will be possible... ever heard of the Pictet-Spengler reaction?
|
|
|
Adas
National Hazard
   
Posts: 711
Registered: 21-9-2011
Location: Slovakia
Member Is Offline
Mood: Sensitive to shock and friction
|
|
Isn't it possible to methylate tryptamine using simply H2SO4 + methanol?
Rest In Pieces!
|
|
|
turd
National Hazard
   
Posts: 800
Registered: 5-3-2006
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by DJF90  | | I dont think alkylation that occurs via an imine will be possible... ever heard of the Pictet-Spengler reaction? |
Maybe it's not possible in theory, but in practice it is.
| Quote: | | Isn't it possible to methylate tryptamine using simply H2SO4 + methanol? |
Doubtful.
|
|
|
smuv
National Hazard
   
Posts: 842
Registered: 2-5-2007
Member Is Offline
Mood: Jingoistic
|
|
A lot of triptan drugs are methylated by reductive alkylations. I think this is a conditions thing. But yes...pictet-spengler can be a competing
reaction.
Edit: haha originally unintelligible.
[Edited on 12-23-2011 by smuv]
"Titanium tetrachloride…You sly temptress." --Walter Bishop
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Thing is, formation of an intermediate imine/iminium cation should react much faster intramolecularly than intermolecularly, although I agree perhaps
there are conditions where the Pictet-Spengler cannot occur. I suspect if you can use conditions which would adversely affect the breaking of
aromaticity in the intramolecular attack then it may be possible. Perhaps thats why typical Eschweiler-Clarke conditions cannot be employed, as I am
sure that reaction proceeds via the P-S pathway.
|
|
|
JibbyDee
Harmless

Posts: 38
Registered: 25-11-2011
Member Is Offline
Mood: No Mood
|
|
I was just looking up naturally occuring tryptamines and came across this one:
Norbaeocystin
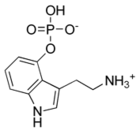
its psilocybin without the N-methyl groups. The wiki page doesn't say much about it but the wiki page for its mono-N-methylated analogue, baeocystin,
states that:
| Quote: |
Little information exists with regard to human pharmacology, but in the book Magic Mushrooms Around the World, author Jochen Gartz reports being aware
of a study in which "10 mg of baeocystin were found to be about as psychoactive as a similar amount of psilocybin." |
Heres another interesting one:
5-Bromo-DMT

According to the wiki page, it occurs naturally in some marine invertebrates. I read that in animal studies, there were indications that it had
sedative and antidepressant properties. This molecule caught my interest because I remember, in a documentary about LSD, hearing about bromo-LSD which
has no psychoactive properties but happens to cure cluster headaches.
Heres a strange one:
Convolutindole A
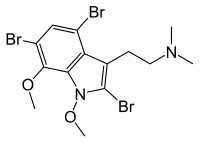
It also occurs in some marine invertebrates. According to the wiki page, it is an effective anti-nematode (parasitic worm) drug.
[Edited on 23-12-2011 by JibbyDee]
|
|
|
497
National Hazard
   
Posts: 778
Registered: 6-10-2007
Member Is Offline
Mood: HSbF6
|
|
Melatonin powder is pretty easy to find too, making all 5-MeO tryptamines available easily. Assymetrically substituted 5-MeO tryptamines should be
easy from melatonin too.
|
|
|
smuv
National Hazard
   
Posts: 842
Registered: 2-5-2007
Member Is Offline
Mood: Jingoistic
|
|
http://www.scribd.com/doc/48650605/Contemporary-Drug-Synthes... Ch12 many examples w. borohydrides/formalin. You can track down primary refs.
Edit:http://pubs.acs.org/doi/pdf/10.1021/jm00018a016
NaBH3CN in acidic MeOH.
[Edited on 12-24-2011 by smuv]
"Titanium tetrachloride…You sly temptress." --Walter Bishop
|
|
|
Methansaeuretier
Harmless

Posts: 45
Registered: 24-5-2010
Location: Europe
Member Is Offline
Mood: strongly alkaline
|
|
Quote: Originally posted by turd  |
What he missed was the sodium triacetoxy borohydride (made in-situ) reductive methylation of tryptamine with formaldehyde to DMT. I have heard
a few success reports. Yields are low in an amateur setting, but given that it's only two steps from (dirt cheap) tryptophan, overall yield
is better than for most OTC routes to phenethylamines.
|
Really? I heard only of one success report wich was with comercial triacetoxyborohydride and I think a fake as triacetoxy borohydride
is extremly sensetive to water and aqueous formaldehyde was used. NaBH(CH3COO)3 or NaBH4/CH3COOH are also able to reduce the indole (see for example
"NaBH4 in carboxylic acid media" on Rhodium archive)! You will get a product but I guess no dialkytryptamines.
Can u link any success reports?
Cold alkaline aquesous NaBH4 solution and aq. formaldehyde should work for formylation by simultaneuos titration (and large excess) into an ice cold
alcoholic solution of the substrate. Or build imine first before adding NaBH4.
A little cross quoting about reductive alkylation:
| Quote: |
Eschweiler-Clarke does not work at all. Picted-Spengler would be the main-reaction.
Also Al/Hg does not work very well/ ~20% yields. Source: Tihkal
NaBH(CH3COO)3 or NaBH4/CH3COOH does also not work/leads to extremly low yields or needs special conditions, because it does reduce indole. Source:
Rhodium archive - several documents on triacetoxyborohydride and NabH4 in carboxylic acid media are mentioning reduction of indoles with it and some
derivates.There are some other reciepes on the in the internet, where this is used to make DMT from tryptamine and aq. Formaldehyde. I think it's pure
a fake. Triacetoxyborohydride is very sensitive to water and does reduce indole - no big chance to work under the mentioned conditions (RT, THF,
excess CH3COOH as solvent).
BH3*THF or NaBH4/H2SO4 or NaBH4/I2 does also not work. The indole gets also reduced very fast under normal conditions.
NaBH4/MeOH . Does work (!!!), but does reduce aldehydes faster than imines/enamines form. It has to be very cold and a large excess of NaBH4 and
aldehyde are neccessary. Does work better for diethylated than for dimethylated tryptamines Source: The Vespiary
H2+Pd/C. Does also work. But needs high pressure of H2. Does sometimes overdo it's job when the catalyst is too strong. Source: Tihkal
Sodium cyaboborohydride does work well but produces side products, esp. beta carbolines. Good workup necessary. Source: Rhodium archive/Vespiary
Untested but very interesting: Zinc borohydride, potassium borohydride and Zn/NaH2PO4
Zinc borohydride because it's known to perfom very well for dialkylations of amines, potassium borohydride because it less active and smoother than
NaBH4 and Zn/NaH2PO4 because it's known to alkylate amino acids and alkylamines in high yields. Zn/NaH2PO4 and formaldehyde does also work for
dialkylation of tryptophane.... but dimethyltryptophane is very hard to get ripped of CO2 and is extremely toxic. Try it yourself.
|
[Edited on 25-12-2011 by Methansaeuretier]
[Edited on 25-12-2011 by Methansaeuretier]
[Edited on 25-12-2011 by Methansaeuretier]
|
|
|
turd
National Hazard
   
Posts: 800
Registered: 5-3-2006
Member Is Offline
Mood: No Mood
|
|
Yes.
| Quote: | | I heard only of one success report wich was with comercial triacetoxyborohydride and I think a fake as triacetoxy borohydride is
extremly sensetive to water and aqueous formaldehyde was used. |
I've seen the analytical data and it's unambigous. Though isolated yield was low (~1 g DMT from 5 g tryptamine).
| Quote: | | NaBH(CH3COO)3 or NaBH4/CH3COOH are also able to reduce the indole (see for example "NaBH4 in carboxylic acid media" on Rhodium archive)!
|
See Chem. Soc. Rev.,27,395(1998).
| Quote: | | Can u link any success reports? |
No, Private Communication. I will not disclose the author, but post some data in due course.
|
|
|
Vogelzang
Banned
Posts: 662
Registered: 26-4-2008
Member Is Offline
Mood: No Mood
|
|
Here's an interesting Schiff base reduction method.

|
|
|
overload
Hazard to Self
 
Posts: 66
Registered: 9-7-2011
Location: USA
Member Is Offline
Mood: miserable fat slave
|
|
Do an old school DMT synthesis and grow the cool looking crystals DMT forms. Don't vaporize them because its neurotoxic to your 5ht2a and you need
those.
|
|
|
Adas
National Hazard
   
Posts: 711
Registered: 21-9-2011
Location: Slovakia
Member Is Offline
Mood: Sensitive to shock and friction
|
|
Quote: Originally posted by overload  | | Do an old school DMT synthesis and grow the cool looking crystals DMT forms. Don't vaporize them because its neurotoxic to your 5ht2a and you need
those. |
DMT is neurotoxic? Really? It occurs naturally in our brains...
Rest In Pieces!
|
|
|
GreenD
National Hazard
   
Posts: 623
Registered: 30-3-2011
Member Is Offline
Mood: Not really high anymore
|
|
From last I heard, the "spearmint" method of decarboxylation was fake (the "Student").
I attempted myself very crudely with a sticky, nasty mess. My skills by no means constitute incapability of the reaction. However I do believe that a
molar ratio of spearmint oil (or molar equivalent of acetophenone) will fully decarboxylate the product... Whether the pictet-spengler occurs, I am
again not sure.
I may continue reading up on this in the future.
I'll report anything.
==Making ethyl bromide is quite easy.
==Obtaining diisopropylethylamine is tricky - but some people can get it.
Tryptophan -> DET may be an easy route for a hobbyist.
|
|
|
Bot0nist
International Hazard
    
Posts: 1559
Registered: 15-2-2011
Location: Right behind you.
Member Is Offline
Mood: Streching my cotyledons.
|
|
I don't believe the OP asked anything about ingestion of tryptamine or its methylated variants. I do hope that the original poster has read these
threads on decarboxylation of tryptophan right here on SciMad. I also saw some patents on the subject IIRC.
<a href="http://www.sciencemadness.org/talk/viewthread.php?tid=8574#pid97905">L-tryptophan decarboxylation</a>
<a href="http://www.sciencemadness.org/talk/viewthread.php?tid=2255#pid23507">decarboxylation of tryptophan</a>
<a href="http://www.sciencemadness.org/talk/viewthread.php?tid=3544#pid39231">tryptophan decarboxylation</a>
*<a href="http://www.sciencemadness.org/talk/viewthread.php?tid=14147#pid182233">Tryptamine refusing to crystallize</a>
*edit: Sorry turd. I missed that good thread when searching. Thanks.
[Edited on 8-1-2012 by Bot0nist]
U.T.F.S.E. and learn the joys of autodidacticism!
Don't judge each day only by the harvest you reap, but also by the seeds you sow.
|
|
|
12332123
Harmless

Posts: 38
Registered: 14-11-2009
Member Is Offline
Mood: No Mood
|
|
I would just use it to enrich cubensis substrate and get some potent-ass shrooms, but that's just me. 
|
|
|
overload
Hazard to Self
 
Posts: 66
Registered: 9-7-2011
Location: USA
Member Is Offline
Mood: miserable fat slave
|
|
Quote: Originally posted by Adas  | Quote: Originally posted by overload  | | Do an old school DMT synthesis and grow the cool looking crystals DMT forms. Don't vaporize them because its neurotoxic to your 5ht2a and you need
those. |
DMT is neurotoxic? Really? It occurs naturally in our brains... |
yes
Edit: On second thought I feel like I should explain the theory ive formed after researching DMT. DMT is a naturally occurring chemical in the brain
as well as plants and possibly other places. It is released during life or death situations or near death experiences and has been thought to be the
cause of people hallucinating when they die but are resuscitated. It may exist as natures way of saying you need to not let that happen again because
it is not a pleasant experience for most people. However.. It's said to produce some euphoria by agonizing 5ht2b receptors and is considered
neurotoxic to these receptors along with others. Its associated with the "I'm going to die" state of mind.
[Edited on 8-1-2012 by overload]
|
|
|
mr.crow
National Hazard
   
Posts: 884
Registered: 9-9-2009
Location: Canada
Member Is Offline
Mood: 0xFF
|
|
On a similar note, what can be done with phenylethylamine? You can buy tons of pure PEA hydrochloride from some supplement website. Nothing naughty of
course.
Double, double toil and trouble; Fire burn, and caldron bubble
|
|
|
| Pages:
1
2
3 |