Spart
Harmless

Posts: 8
Registered: 18-12-2011
Member Is Offline
Mood: No Mood
|
|
Diamond may not be hardest natural substance.
I found this small article pretty interesting. I have yet to research into it a little more deeply, but I thought it was pretty interesting that
Diamond may not be the hardest natural substance on Earth because it is indeed widely regarded as such.
http://www.newscientist.com/article/dn16610-diamond-no-longe...
This article was produced in 2009, so perhaps they were proven wrong, but for all I have found so far, they could be right. It's odd because I have
never been taught anything other than Diamond being the hardest natural substance of Earth. As far as I know, it seems to be widely unknown that it
may not be the hardest natural substance on Earth,
If anyone could provide some insight or information on this topic that would be great.
[Edited on 21-12-2011 by Spart]
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Several years ago I actually tore apart my chemistry professor's model of the lattice structure of diamond and rearranged it into a "new hypothetical
allotrope of carbon", and speculated to my teacher about how crystals of such a new allotrope could be formed (I proposed growing them on a different
compound with a similar crystal structure). So now I learn that this hypothetical compound I imagined actually exists- "Lonsdaleite" (hexagonal
diamond), which has a wurtzite structure (conveniently for me, there was also a lattice structure of wurtzite next to my teacher's diamond structure,
this in fact was what gave me the idea to begin with  ). Apparently wikipedia
did not have an entry for "Lonsdaleite" at that time. ). Apparently wikipedia
did not have an entry for "Lonsdaleite" at that time.
Thanks Spart for bringing this to my attention! 
The interesting thing about the lattice structure of hexagonal diamond is that it appears virtually identical to diamond when a model is made. The
difference in geometry is very subtle. The best way I can describe the difference is to take two carbon atoms, from a "ball and stick" kit, stuck
together and studed with sticks in the normal tetrahedral bond angles for tetravalent carbon. Rotate the two carbon atoms relative to eachother. When
the sticks from the two carbon atoms "overlap", this is the "hexagonal diamond" structure. When the sticks from the two carbon atoms are in "opposite"
orientations, this is the configuration found in "normal diamond", the latter of which is obviously more favorable.
The problem seems to be actually making Lonsdaleite in more than microscopic quantities.
[Edited on 21-12-2011 by AndersHoveland]
|
|
|
fledarmus
Hazard to Others
  
Posts: 187
Registered: 23-6-2011
Member Is Offline
Mood: No Mood
|
|
Wow - essentially an "all boat" conformation of cyclohexane rings, instead of the adamantite "all chair" conformation! An interesting thought...
|
|
|
White Yeti
National Hazard
   
Posts: 816
Registered: 20-7-2011
Location: Asperger's spectrum
Member Is Offline
Mood: delocalized
|
|
β-C<sub>3</sub>N<sub>4</sub> is a hypothetical substance predicted to be harder than diamond. Unfortunately it's a
hypothetical substance, that is, even more expensive than diamond. Here's what it looks like:
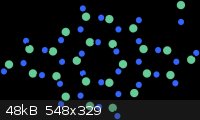
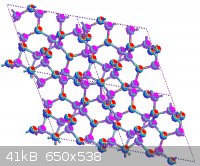
As a tangent, boron sub-oxide is also very interesting diamond-like substance. As a matter of fact, it may be the easiest diamond-like substance for
the amateur to make in a lab (relatively speaking).
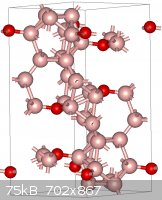
"Ja, Kalzium, das ist alles!" -Otto Loewi
|
|
|
ibro
Harmless

Posts: 14
Registered: 28-3-2012
Member Is Offline
Mood: No Mood
|
|
I still think it is..
|
|
|
neptunium
National Hazard
   
Posts: 990
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
in nature (not limited to earth) there is other harder things than diamonds.
a neutron star contain degenarate matter as dense as a nuclear core . it would be (if it could be handled) harder much denser and heavier than
diamond.
|
|
|
White Yeti
National Hazard
   
Posts: 816
Registered: 20-7-2011
Location: Asperger's spectrum
Member Is Offline
Mood: delocalized
|
|
Quote: Originally posted by neptunium  | in nature (not limited to earth) there is other harder things than diamonds.
a neutron star contain degenarate matter as dense as a nuclear core . it would be (if it could be handled) harder much denser and heavier than
diamond. |
Maybe denser, but harder? I'm not so sure. According to wikipedia, particles in a neutron star are held together by gravity, the weakest force of them
all...
"Ja, Kalzium, das ist alles!" -Otto Loewi
|
|
|
White Yeti
National Hazard
   
Posts: 816
Registered: 20-7-2011
Location: Asperger's spectrum
Member Is Offline
Mood: delocalized
|
|
Pertinent articles of potential interest to the discussion:
http://phys.org/news/2011-10-superhard-carbon.html
http://phys.org/news/2010-11-super-hard-carbon.html
"Ja, Kalzium, das ist alles!" -Otto Loewi
|
|
|
turd
National Hazard
   
Posts: 800
Registered: 5-3-2006
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by White Yeti  | | Maybe denser, but harder? I'm not so sure. According to wikipedia, particles in a neutron star are held together by gravity, the weakest force of them
all... |
WTF am I reading!? This must be one of the most illogical things ever posted on this site. From the above mentioned article:
| Quote: | | The pressure increases from 0.3 to 16×1034 Pa from the inner crust to the center.[16] |
!
|
|
|
White Yeti
National Hazard
   
Posts: 816
Registered: 20-7-2011
Location: Asperger's spectrum
Member Is Offline
Mood: delocalized
|
|
If you think that just because something is incredibly dense that it will also be extremely hard, then mercury should be harder than elemental boron.
We cannot agree upon the hardness of neutron stars because we don't even know what the center is made up of. Even then, which part of the star do you
want to consider? The crust, the outer core, the core?
You can't even MEASURE the hardness of a neutron star. You can give an approximation, but that's about it.
"Ja, Kalzium, das ist alles!" -Otto Loewi
|
|
|
turd
National Hazard
   
Posts: 800
Registered: 5-3-2006
Member Is Offline
Mood: No Mood
|
|
Jeez. It's hard, but I will refrain from bringing the obvious pun. 
You didn't even understand what I said. When I say your posting is unbelievably illogical, then I'm attacking your reasoning not your
conclusion (not that it is a correct conclusion). A conclusion can only be right or wrong. Yet, you answered by defending your conclusion instead of
your reasoning - with even more illogical nonsense.
Quote: Originally posted by White Yeti  | | If you think that just because something is incredibly dense that it will also be extremely hard, then mercury should be harder than elemental boron.
|
Nobody said that neutron stars are hard because they are dense. That's an argument YOU made up. What you did is called attacking a straw man and it's
lame and transparent.
You said that you think that neutron stars are not hard because gravity is a weak force, with reference to Wikipedia. But the same article (did you
read it?) said that the pressure in a neutron star is on the order of 10<sup>34</sup> Pa(sic!). In case you fail to realize it: pressure
is force per surface. The forces in a neutron star are absolutely incredible. In consequence your reasoning is stupid.
| Quote: | | You can't even MEASURE the hardness of a neutron star. |
So what? Just because something can't be measured directly it does not exist? That's silly and you certainly don't want to start a discussion on
epistemology given your track record in drawing conclusion. (Note: the whole thread is about materials according to simulations - neutron
stars certainly are more real than that.).
If there are parts in a neutron star that behave somehow like a solid, then this matter withstands friggin' 10<sup>34</sup> Pa. Do you
seriously believe you could put a dent in that by applying a force that wouldn't totally demolish any diamond on earth? So whatever your definition of
hardness is - this matter is certainly orders of magnitude harder.
| Quote: | | You can give an approximation, but that's about it. |
More nonsense. Is that seriously supposed to be an argument? In physics everything is an approximation. If value a is approximated to 1±0.01
and value b is approximated to 1010±5, then b still is orders of magnitude larger than a, despite being only
approximated very roughly.
Honestly, this is your problem: https://en.wikipedia.org/wiki/Dunning%E2%80%93Kruger_effect.
Before learning physics, try to learn how to construct an argument. Nothing in your posting substantiated your position. It was just random, unsorted
thoughts.
|
|
|
White Yeti
National Hazard
   
Posts: 816
Registered: 20-7-2011
Location: Asperger's spectrum
Member Is Offline
Mood: delocalized
|
|
Quote: Originally posted by turd  |
You said that you think that neutron stars are not hard because gravity is a weak force, with reference to Wikipedia. But the same article (did you
read it?) said that the pressure in a neutron star is on the order of 10<sup>34</sup> Pa(sic!). In case you fail to realize it: pressure
is force per surface. The forces in a neutron star are absolutely incredible. In consequence your reasoning is stupid. |
I said that gravity is a weak force as compared to all the other fundamental forces. Compare gravity to the strong force that keeps hadrons together.
I'm willing to bet that individual baryons are "harder" than any part of a neutron star because of the tremendous strength of the strong force
(1039 stronger than gravity).
"Ja, Kalzium, das ist alles!" -Otto Loewi
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by White Yeti  | | I'm willing to bet that individual baryons are "harder" than any part of a neutron star because of the tremendous strength of the strong force
(1039 stronger than gravity). |
Hardness is an attribute of solid materials. How is it that you
have come to suppose that either hadrons or neutron star materials are in a solid phase?
|
|
|
turd
National Hazard
   
Posts: 800
Registered: 5-3-2006
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by White Yeti  | | I said that gravity is a weak force as compared to all the other fundamental forces. Compare gravity to the strong force that keeps hadrons together.
I'm willing to bet that individual baryons are "harder" than any part of a neutron star because of the tremendous strength of the strong force
(1039 stronger than gravity). |
Again, you are trying to weasel out of the situation by completely misrepresenting what you said. This is not very smart, since your posting is still
visible on the same page:
| Quote: | | Maybe denser, but harder? I'm not so sure. |
You disputed the assertion that neutron start are harder than diamonds.
| Quote: | | According to wikipedia, particles in a neutron star are held together by gravity, the weakest force of them all... |
And your reasoning was that gravity is a weak force. This reasoning is silly and proves a complete lack of grasp of physics on your part.
Now that isn't terrible per se - everybody has a brain fart every once a while and started as a noob. But your complete lack of self-critisism and the
extreme overrating of your own abilities is annoying. Both make you useless as a scientist and you should try to get rid of these attributes as soon
as possible. But the worst is the way you try to turn around your arguments. That's downright offensive: it's assuming that your dialog partner is
stupid. You can try these stunts with your high-school peers, but not here.
|
|
|
White Yeti
National Hazard
   
Posts: 816
Registered: 20-7-2011
Location: Asperger's spectrum
Member Is Offline
Mood: delocalized
|
|
Quote: Originally posted by turd  | | Now that isn't terrible per se - everybody has a brain fart every once a while and started as a noob. But your complete lack of self-critisism and the
extreme overrating of your own abilities is annoying. Both make you useless as a scientist and you should try to get rid of these attributes as soon
as possible. But the worst is the way you try to turn around your arguments. That's downright offensive: it's assuming that your dialog partner is
stupid. |
Yes, I know what I say is not brilliant. I have a hard time admitting it. FYI I do criticise myself and I don't hold up my arguments to a high esteem
either, I'm just trying not to lose face. Apparently, I've been doing the opposite. I'm not assuming anyone is stupid, if I really wanted to argue
with stupid people, I'd pick on people in another forum.
I apologise.
"Ja, Kalzium, das ist alles!" -Otto Loewi
|
|
|
turd
National Hazard
   
Posts: 800
Registered: 5-3-2006
Member Is Offline
Mood: No Mood
|
|
No need to apologize - stupid is probably one of the nicer things I've been called here. 
At the risk of sounding pompous: The road to success is paved with failure. 
|
|
|
hyfalcon
International Hazard
    
Posts: 1003
Registered: 29-3-2012
Member Is Offline
Mood: No Mood
|
|
Space elevator anyone?
|
|
|
zed
International Hazard
    
Posts: 2284
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Beam me up Scotty!
Me and the boys down at the feed store, have always assumed that Boron Nitride was a lot harder than diamond. Turns out it was, sort of.
Also, the way I remember it, Boron Nitride might be water soluble.
So, as hard as it is, its usefulness might be limited.
http://arstechnica.com/science/2009/02/boron-nitride-harder-...
[Edited on 4-10-2012 by zed]
[Edited on 4-10-2012 by zed]
|
|
|
Oscilllator
National Hazard
   
Posts: 659
Registered: 8-10-2012
Location: The aqueous layer
Member Is Offline
Mood: No Mood
|
|
Time to revive a dead thread!
How would that make it not useful? you could dissolve it in water than precipitate it out and get razor-sharp crystals, or you could coat a drill-bit
etc. having something harder than diamond that was water-soluble would be way more useful!
|
|
|