Magelia
Harmless

Posts: 28
Registered: 26-10-2011
Member Is Offline
Mood: No Mood
|
|
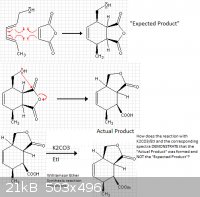
Diels-Alder Reaction THEN Intramolecular Reaction
Hello Colleagues,
So, I am faced with a dilemma. I did a diels-alder reaction between maleic anhydride and trans,trans,2-4-hexadiene-1-ol and I was expected to form the
normal diels-alder compound that is seen when those two compounds react (Diagram shown below). However, I knew the OH could do an intramolecular
reaction therefore the ACTUAL product of my reaction was the cyclic ester... the anhydride moiety was gone.
So, to confirm that the intramolecular reaction did happen... I reacted my product of maleic anhydride and trans,trans,2-4-hexadiene-1-ol with
potassium carbonate and ethyl iodide with 2-butanone as a solvent and I got the spectrum shown below.
The thing is, HOW does the reaction I did with ethyl iodide and potassium explain that the product I obtained from the reaction of maleic anhydride
and trans,trans,2-4-hexadiene-1-ol is not the "expected" product but the product AFTER the intramolecular nucleophilic attack of the OH at the
carbonyl forming a cyclic ester? How do I explain that my reaction conditions of K2CO3/EtI PROVES/DEMONSTRATES that I did indeed get the cyclic ester
product!
Any help would be very very appreciated! 
Attachment: Spectra of K2CO3_EtI reaction.pdf (56kB)
This file has been downloaded 652 times
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Try the ethylation conditions with maleic or succinic anhydride - it shouldnt work, only for free acids. If it doesn't work, then you must have had a
free acid in your product, pointing to intramolecular attack. You could also show your product is not the expected anhydride by MS - there should be a
mass difference between the hydroxy-anhydride and the lactone-acid.
|
|
|
ziqquratu
Hazard to Others
  
Posts: 385
Registered: 15-11-2002
Member Is Offline
Mood: No Mood
|
|
The NMR is proof enough - for the expected product, with the free alcohol and intact anhydride, the CH2OH protons will be MUCH further upfield
than the corresponding protons in the actual product (my estimate is 3.5 ppm for the CH2 in the alcohol, vs 4-ish ppm for the CH2 in the ester).
As far as the ethylation goes, the same argument applies. Ethylation of the free alcohol of the expected product would give an ether, with peaks for
both methylenes at around 3.5 ppm; methylation of the ester product would result in the formation of a diester, with two sets of CH2 peaks around 4
ppm.
|
|
|
fledarmus
Hazard to Others
  
Posts: 187
Registered: 23-6-2011
Member Is Offline
Mood: No Mood
|
|
I think the point that you are trying to demonstrate is that there is no free alcohol in your product. If there was an alcohol, it would have made an
ether under these conditions, as Zigguratu pointed out.
|
|
|
Magelia
Harmless

Posts: 28
Registered: 26-10-2011
Member Is Offline
Mood: No Mood
|
|
Guys... YOU DA BEST!
Great explanation. Clear and concise and to the point! 
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Magelia  | | So, I am faced with a dilemma. I did a diels-alder reaction between maleic anhydride and trans,trans,2-4-hexadiene-1-ol and I was expected to form the
normal diels-alder compound that is seen when those two compounds react (Diagram shown below). However, I knew the OH could do an intramolecular
reaction therefore the ACTUAL product of my reaction was the cyclic ester... the anhydride moiety was gone. |
Why do you think the acylation is intramolecular and that it occurs after the Diels-Alder? You provided no experimental details, but I would guess you
heated the reaction mixture somewhere in between 120 and 200 °C, which is enough for the maleic anhydride to acylate the dienyl alcohol even before
the Diels-Alder reaction starts. It is thus just as possible that it is the Diels-Alder that is intramolecular, rather than the acylation. The rate of
intramolecular Diels-Alders is much faster than the intermolecular ones (when geometry allows).
| Quote: | | So, to confirm that the intramolecular reaction did happen... I reacted my product of maleic anhydride and trans,trans,2-4-hexadiene-1-ol with
potassium carbonate and ethyl iodide with 2-butanone as a solvent and I got the spectrum shown below. |
You might want to elaborate your thought on how this ethylation is supposed to demonstrate anything. Even if you obtained the so called "expected
product" it would rapidly rearrange to the "actual product" under K2CO3 treatment. The ethylation reaction products from both would therefore be the
same!
| Quote: | | The thing is, HOW does the reaction I did with ethyl iodide and potassium explain that the product I obtained from the reaction of maleic anhydride
and trans,trans,2-4-hexadiene-1-ol is not the "expected" product but the product AFTER the intramolecular nucleophilic attack of the OH at the
carbonyl forming a cyclic ester? How do I explain that my reaction conditions of K2CO3/EtI PROVES/DEMONSTRATES that I did indeed get the cyclic ester
product! |
Unfortunately, due to the mentioned reason, it can demonstrate absolutely nothing.
Like ziqquratu said, the 1H NMR spectra of the product would be enough, but you did not provide that spectra, so it is not up to us to draw
conclusions.
Quote: Originally posted by DJF90  | | Try the ethylation conditions with maleic or succinic anhydride - it shouldnt work, only for free acids. If it doesn't work, then you must have had a
free acid in your product, pointing to intramolecular attack. You could also show your product is not the expected anhydride by MS - there should be a
mass difference between the hydroxy-anhydride and the lactone-acid. |
The anhydride and the lactone would give the same HRMS result!
Also, maleic anhydride can not rearrange to a lactone under the same ethylation conditions (it has no alcohol group), so the absence of a reaction
would prove nothing.
Quote: Originally posted by fledarmus  | | I think the point that you are trying to demonstrate is that there is no free alcohol in your product. If there was an alcohol, it would have made an
ether under these conditions, as Zigguratu pointed out. |
If there was a free alcohol as in the "expected product", then this compound would rearrange into the lactone (see the cis configuration
dictating a rearrangement), the carboxylate of which would then be ethylated to the same ester.
Also, with K2CO3 you can have a chemoselective O-alkylation of the carboxylate and no O-alkylation of the alcohol function (the absence of the ethyl
ether therefore demonstrates nothing). It depends on the reaction solvent, time and temperature, but it is not easy to O-alkylate alcohols using K2CO3
as a base.
No, we all lousy, the explanation was no good, totally missed the point, and you would gain a lot by learning some critical thinking. Otherwise,
welcome to the forum.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Magelia
Harmless

Posts: 28
Registered: 26-10-2011
Member Is Offline
Mood: No Mood
|
|
Well Nicoderm, I forgot to include some important experiment details.
I first did my Diels-Alder reaction between maleic anhydride and trans,trans,2-4-hexadiene-1-ol and took its IR and H-NMR Spectrum. I then went home,
watched some soccer on TV, and went to bed.
The next day I went back to the lab and did the ethylation on my Diels-Alder product and got its H-NMR Spectrum that I had posted.
Therefore, the earlier mentioned points by the other authors did indeed make a lot of sense and were correct.
Thank you though for your answers, they were well said.
Of course, thank you for welcoming me to the forum  . .
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Well, how about sharing the information? You get us interested and then you don't tell us the conclusion you got from the NMR. In the context of my
cultural background, that is quite an impolite behavior.
|
|
|
turd
National Hazard
   
Posts: 800
Registered: 5-3-2006
Member Is Offline
Mood: No Mood
|
|
Interesting: The original poster is unable to look up answers to questions like https://www.sciencemadness.org/whisper/viewthread.php?tid=17... in a book, but has access to weird dienes and time on the NMR spectrometer?
|
|
|
Magelia
Harmless

Posts: 28
Registered: 26-10-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Nicodem  | | Well, how about sharing the information? You get us interested and then you don't tell us the conclusion you got from the NMR. In the context of my
cultural background, that is quite an impolite behavior. |
Sorry Nicodem, I am still learning. I'll try to do my best next time I post/say something.
Thanks for the investigative work, and trying to put me down. Appreciate it.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
I just wanted to know whether the NMR confirmed the anhydride-type or the lactone-type of the product? I find it hard to deduce anything in this
regard from what you wrote up to now. I guess you found out it was the lactone?
If you refuse to give an answer because you are still unsure, then I suggest you to post the spectra and we'll determine what it is together.
|
|
|
Magelia
Harmless

Posts: 28
Registered: 26-10-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Nicodem  |
I just wanted to know whether the NMR confirmed the anhydride-type or the lactone-type of the product? I find it hard to deduce anything in this
regard from what you wrote up to now. I guess you found out it was the lactone?
If you refuse to give an answer because you are still unsure, then I suggest you to post the spectra and we'll determine what it is together.
|
Well, again I forgot to mention I got the IR spectrum of my product as well  So
based on that it was pretty easy to see what type of carbonyl I had So
based on that it was pretty easy to see what type of carbonyl I had 
|
|
|