| Pages:
1
2 |
Chemistry Alchemist
Hazard to Others
  
Posts: 403
Registered: 2-8-2011
Location: Australia
Member Is Offline
Mood: No Mood
|
|
dechlorinating 1,4-Dichlorobenzene
Question - How could you turn 1,4-Dichlorobenzene into benzene?
1,4-Dichlorobenzene is a chemical substitute for Naphthalene moth balls, so it could be easy to get for the home chemist, what would you have to do to
remove the chlorine from it? what would be the byproducts?
[Edited on 30-10-2011 by Chemistry Alchemist]
|
|
|
BromicAcid
International Hazard
    
Posts: 3247
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Organic Syntheses, Vol. 47, p. 103 (1967); Coll. Vol. 5, p.998 (1973).
You can view it at the orgsyn.org website since I have trouble with direct links due to their disclaimer and use of frames.
|
|
|
Chemistry Alchemist
Hazard to Others
  
Posts: 403
Registered: 2-8-2011
Location: Australia
Member Is Offline
Mood: No Mood
|
|
I cant seem to find the section.
|
|
|
BromicAcid
International Hazard
    
Posts: 3247
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Just enter the information on the main page and click 'go'. The procedure is for the reduction of chlorobenzene to benzene but this can be expanded
to other aromatic halides.
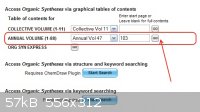
|
|
|
Chemistry Alchemist
Hazard to Others
  
Posts: 403
Registered: 2-8-2011
Location: Australia
Member Is Offline
Mood: No Mood
|
|
sorry if i sound like a noob but i still cant find it 
|
|
|
querjek
Hazard to Self
 
Posts: 76
Registered: 26-8-2008
Member Is Offline
Mood: No Mood
|
|
This topic has been discussed a few times already. Search for "dichlorobenzene" or "paradichlorobenzene".
it's all about chemistry.
|
|
|
bbartlog
International Hazard
    
Posts: 1139
Registered: 27-8-2009
Location: Unmoored in time
Member Is Offline
Mood: No Mood
|
|
I'm going to guess that you're searching on 'dichlorobenzene', which will not bring up the relevant item. Try 'reduction of organic halides'. That
will give you the article BromicAcid mentions as the only hit.
|
|
|
Chemistry Alchemist
Hazard to Others
  
Posts: 403
Registered: 2-8-2011
Location: Australia
Member Is Offline
Mood: No Mood
|
|
Do u search up "Reduction of Organic Halides" on the search on this forum or on the site BromicAcid said?
|
|
|
Chemistry Alchemist
Hazard to Others
  
Posts: 403
Registered: 2-8-2011
Location: Australia
Member Is Offline
Mood: No Mood
|
|
Would LiAlH<sub>4</sub> work reducing the 1,4-Dichlorobenzene to benzene? i found a website and it says its used in alot of organic
reactions as a reducing agent...
|
|
|
Chemistry Alchemist
Hazard to Others
  
Posts: 403
Registered: 2-8-2011
Location: Australia
Member Is Offline
Mood: No Mood
|
|
How could i just make Chlorobenzene from 1,4-Dichlorobenzene?
|
|
|
Chemistry Alchemist
Hazard to Others
  
Posts: 403
Registered: 2-8-2011
Location: Australia
Member Is Offline
Mood: No Mood
|
|
The process on the website BromicAcid said above is the reduction od organic halides, it only mentions chlorobenzene not 1,4-Dichlorobenzene... So
what I thought instead of trying to make benzene from it, how could I brake it down to chlorobenzene? I can then make phenol from it.
|
|
|
497
National Hazard
   
Posts: 778
Registered: 6-10-2007
Member Is Offline
Mood: HSbF6
|
|
How about this? http://en.m.wikipedia.org/wiki/Poly(p-phenylene_sulfide)
Then hydrogenolysis with raney nickel should yield benzene. Easy as that...
[Edited on 3-11-2011 by 497]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
How?
|
|
|
barley81
Hazard to Others
  
Posts: 481
Registered: 9-5-2011
Member Is Offline
Mood: No Mood
|
|
Base-catalyzed hydrolysis, but it was said previously somewhere in a thread that the hydrolysis needs impractical conditions.
Quote: (user not_important):
The Raschig phenol process is little used nowadays, having fallen out of favour in the 1970s. As it takes place in the vapour phase at temperatures in
the low-to-mid 400 C range, and with incomplete conversion, it is not too convenient for amateur application; it really is an industrial scale
process.
[Edited on 3-11-2011 by barley81]
|
|
|
Chemistry Alchemist
Hazard to Others
  
Posts: 403
Registered: 2-8-2011
Location: Australia
Member Is Offline
Mood: No Mood
|
|
Phenol is made by reacting C<sub>6</sub>H<sub>5</sub>Cl + NaOH = C<sub>6</sub>H<sub>6</sub>O + NaCl
Sorry if the equation is unbalanced, just woke up
[Edited on 4-11-2011 by Chemistry Alchemist]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
I asked how can you make phenol from chlorobenzene. I did not ask you to write an equation. Do you seriously think I can not write equations myself?
(Meanwhile your equation makes no sense chemistry-wise, even though it is balanced.)
The answer to my question should as a minimum contain at least one pertaining reference followed by the explanation on how are you
going to replicate the synthesis. It is absurd that I have to explain on how to structure a reply to my very simple question.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Chemistry Alchemist
Hazard to Others
  
Posts: 403
Registered: 2-8-2011
Location: Australia
Member Is Offline
Mood: No Mood
|
|
Oh sorry, the method is via this route: This is a easy reason. All we need to do is react it with a strong base, the easiest base to get out hands on
is sodium hydroxide so that's what we will use. Start by getting a round bottom flask and set up for heating, now place in 39.99 grams of Sodium
Hydroxide and add to that 112.55 grams of Chlorobenzene. heat the 2 chemicals together, now we wont really know when the reaction is complete because
there will be no indicator, but we could add a few drops of Phenolphthalein to the reaction vessel.. the solution will be deep purple as it will be
really basic but will turn clear as the strong base is used up and reacted telling up its complete. Once complete, take it off heat and let it
evaporate or continue boiling until dry. Now we have 2 products, Sodium Chloride and Phenol. the best way to separate the 2 will be acetone. this is a
great way of separating because Phenol is easy soluble in the acetone while Sodium Chloride is practically insoluble (only 0.00042g/100ml of acetone).
once fully mixed in filter off the Sodium chloride and then poor the acetone into a wide dish for faster drying, now you should have a reasonably pure
Phenol...
I read up on dehalogenation of organic helides and it says reacting them with strong bases but it says it doesnt work with chlorobenzene... is this
the same as 1,4 dichlorobenzene?
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Chemistry Alchemist  | | Oh sorry, the method is via this route: This is a easy reason. All we need to do is react it with a strong base, the easiest base to get out hands on
is sodium hydroxide so that's what we will use. Start by getting a round bottom flask and set up for heating, now place in 39.99 grams of Sodium
Hydroxide and add to that 112.55 grams of Chlorobenzene. heat the 2 chemicals together, now we wont really know when the reaction is complete because
there will be no indicator, but we could add a few drops of Phenolphthalein to the reaction vessel.. the solution will be deep purple as it will be
really basic but will turn clear as the strong base is used up and reacted telling up its complete. Once complete, take it off heat and let it
evaporate or continue boiling until dry. Now we have 2 products, Sodium Chloride and Phenol. the best way to separate the 2 will be acetone. this is a
great way of separating because Phenol is easy soluble in the acetone while Sodium Chloride is practically insoluble (only 0.00042g/100ml of acetone).
once fully mixed in filter off the Sodium chloride and then poor the acetone into a wide dish for faster drying, now you should have a reasonably pure
Phenol... |
I will heavily restrain myself, so I will only ask you to give us the references, please?
|
|
|
bbartlog
International Hazard
    
Posts: 1139
Registered: 27-8-2009
Location: Unmoored in time
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Chemistry Alchemist  | Oh sorry, the method is via this route: [...]
I read up on dehalogenation of organic helides and it says reacting them with strong bases but it says it doesnt work with chlorobenzene... is this
the same as 1,4 dichlorobenzene? |
You are making stuff up. Nicodem is obviously aware that you have no reference for your flight of fancy. You don't even really seem to understand that
there is a difference between reductive dehalogenation (which was what you initially asked for) and dehalogenation via basic hydrolysis, which
replaces the halide with a hydroxyl group.
Anyway, if you actually care, here is a nice ACS overview from 1928: http://pubs.acs.org/doi/abs/10.1021/ie50218a006
Notice that in order to turn chlorobenzene into phenol via aqueous base you'll be wanting a pressure vessel, temps of 300C or thereabouts, and likely
a copper catalyst to speed things along.
I also would like to point out that instead of trying to make any use of the answers that people have provided, you just keep changing the question.
How about actually trying one of the many things you've proposed?
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Samarium(II) Iodide probably would reduce it.
http://en.wikipedia.org/wiki/Reductions_with_samarium(II)_iodide
|
|
|
Chemistry Alchemist
Hazard to Others
  
Posts: 403
Registered: 2-8-2011
Location: Australia
Member Is Offline
Mood: No Mood
|
|
Just my luck, I'm in the process of extracting samarium from a magnet
|
|
|
jon
Hazard to Others
  
Posts: 459
Registered: 11-1-2006
Member Is Offline
Mood: paranoid distrustful apprehensive
|
|
ammonium formate/palladium on carbon, and methanol is a good way to dechlorinate aryl chlorides.
yeilds are quantitative, the reaction is done at room temp in 3 hours with stirring under inert atmosphere and, the reduction is chemoselective, it
tolerates benzylic esters and alcohols, perfect for dechlorinating loperamide to something useful.
ratios are 5% molar palladium (loading 10% but 5 and 3% also work with a little heat input)
and 2 moles ammonium formate per mole chlorine.
[Edited on 22-11-2011 by jon]
[Edited on 22-11-2011 by jon]
Give me librium or give me meth!
Patrick Henry....
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
@Dear bbatlog,
Accoding to this patent you think using Copper Catalyst has same effect in hydrolysis of P-Chlorotoluene to cresol(Para and meta)?
[Edited on 24-11-2011 by Waffles SS]
|
|
|
bbartlog
International Hazard
    
Posts: 1139
Registered: 27-8-2009
Location: Unmoored in time
Member Is Offline
Mood: No Mood
|
|
That's not a patent, and it makes no claims about what I think. And I don't know the answer to the question that I *think* you asked, which is whether
copper would be a viable catalyst for the reaction that is described. It would definitely be worth trying, though, since NaOH at 350C is no joke and
copper would at least give you a chance at conducting the reaction at a somewhat lower temperature.
|
|
|
Chemistry Alchemist
Hazard to Others
  
Posts: 403
Registered: 2-8-2011
Location: Australia
Member Is Offline
Mood: No Mood
|
|
I'm sorry if we have already discussed this and sorry if it's wrong..l I'm just guessing.... Blame the caffeine if I'm wrong  would NaOH have any effect to 1-4 Dichlorobenzene? Would it produce chlorobenzene or
not? Just a random thought... would NaOH have any effect to 1-4 Dichlorobenzene? Would it produce chlorobenzene or
not? Just a random thought...
|
|
|
| Pages:
1
2 |