CaliusOptimus
Hazard to Self
 
Posts: 96
Registered: 10-6-2011
Member Is Offline
Mood: Subjectively Objective
|
|
Distillation of H2SO4
i tried distilling some h2so4 today. i added 250mL of the rooto acid to a 500mL rbf, with a stir bar and 500mL splash head. i chilled the condenser
and receiving flask to -5C. i insulated the splash head and still head with fiberglass, and pulled pressure down to around 25 microns (3.3x10e-2mBar).
i slowly raised the temp to 150C with aggressive stirring. after all that, i still wasnt able to get the acid to boil. there was a little out-gassing
as the temp went up, but that mostly ceased as the temp stabilized at 150C. i checked my vac lines for kinks, and i used a brand new 2stage vane pump.
i also let it sit at 150C for about 10 min to make sure my pump had pulled its max pressure and still, nothing. my rig held vacuum without the pump,
so i know there arent any significant leaks.
according to the BP calc i should be distilling some acid right now. whats going on?
|
|
|
Steve_hi
Hazard to Others
  
Posts: 196
Registered: 4-12-2010
Member Is Offline
Mood: No Mood
|
|
Does this help? do you know what the concentration of your H2SO4 is? looks like you need to be closer to 200°c
[img]C:\Users\Steve\Pictures\boiling-point_e.jpg[/img]
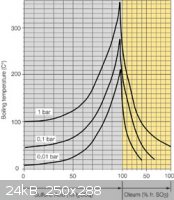
[Edited on 16-10-2011 by Steve_hi]
|
|
|
BromicAcid
International Hazard
    
Posts: 3247
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
From the "Boiling Point Calculator" page:
| Quote: | The results are only roughly evaluated and
can sometimes differ a bit from their real values. |
The calculator gives results for more ideal liquids, sometimes they can be far from the truth.
Also, where were you measuring the pressure? That's an awful good vacuum, you use a McCloud Gauge to read it? Did you measure before the traps,
after the traps, on the pot, at the pump? Those can all make a big difference. Plenty of times I've been at 0.1 mmHg at the pump (100 microns if the
conversion calculator is correct) but at the pot I'll be closer to 3.0 mmHg, not due to vacuum loss but due to the vapors in the headspace. Once it
starts to go over the two numbers start to come to equilibrium but there can be major descripencies. Also, why did you chill the receiver so much?
You shouldn't have to chill at all, when I distilled H2SO4 (albeit atmospherically) I ran air through my condensers for fear of shattering them.
Did you have some reason that you didn't just try to go hotter? Sometimes things need that extra push to get them started.
|
|
|
Steve_hi
Hazard to Others
  
Posts: 196
Registered: 4-12-2010
Member Is Offline
Mood: No Mood
|
|
[img]C:\Users\Steve\Pictures\Capture2.jpg[/img]

|
|
|
CaliusOptimus
Hazard to Self
 
Posts: 96
Registered: 10-6-2011
Member Is Offline
Mood: Subjectively Objective
|
|
that was quite a helpful response bromicacid. thanks!
the reason i didnt go hotter was because my stirplate takes ages to reach higher temps, and i didnt want to put that much of a temp differential on my
condenser. i kept the water so cold to help keep vapors away from my gauge, and my nice new pump. plus my trap was NaOH and that makes water vapor
which pretty much murders the performance on a rotary vane pump. hence, the less vapor the better. that pressure was roughly measured with a
mechanical gauge at the vac adapter. i also know my pump can pull that. i didnt think of measuring at the pot, thats a good call. ive got pirani
gauges but i wont use those without a good trap. im thinking about what you said about the vapors.....i think a trap would remove the vapors and also
their partial pressure. what do you think?
|
|
|
Erbium_Iodine_Carbon
Harmless

Posts: 41
Registered: 6-8-2011
Location: ON, Canada
Member Is Offline
Mood: No Mood
|
|
Even if a bit of water is formed in the NaOH trap, sodium hydroxide is very hydroscopic and will absorb the water. In fact, I've used NaOH in an
impromptu dessicator jar before with success. Furthermore, concentrated H2SO4 will pull water out of the air very quickly. All this to say that you
probably don't need that much cooling.
Also, in my experiences with distilling drain cleaner, I found that the first bit of liquid that is pulled over is mostly water (95% concentrated goes
up to 98% with little acid loss). I was heating my acid over a blowtorch (I don't have nearly the same equipment as you do) and I found that once the
acid was concentrated, it would all condense even before the condenser and run back into the boiling flask. I had to do a wierd set-up which involved
tilting the boiling flask so the arm was angled towards the condenser. Another way to tell if the acid is concentrated is put a piece of paper into
the fumes that are boiling off. If the paper immediately turns black, the acid is concentrated enough to start collecting it (I actually concentrated
my acid after distilling it, but the same principle should work before).
|
|
|