| Pages:
1
2
3 |
D4RR3N
Hazard to Others
  
Posts: 271
Registered: 9-1-2007
Member Is Offline
Mood: No Mood
|
|
aluminium oxide crystal
How can I grow an aluminium oxide crystal without the need of actually melting aluminium oxide (melts at over 2000C!)
What temperature would aluminium hydroxide decompose into aluminium oxide?
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
One source notes that by passing NH3 into an aqueous Al salt solution, a voluminous amorphous substance called Aluminum oxide hydrate is formed. This
slowly changes via boehmite and bayerite into the thermodynamically stable hydragillite.
Gamma Al2O3 can then be prepared by the cautious dehydration of hydragillite or boehmite. Heating over 1000 C produces Alpha Al2O3, noted for its
hardness.
Alpha Al(OH)3 (which can be dehydrated to Al2O3, I would guess the Alpha form) can be directly prepared by passing CO2 through Sodium aluminate
solution at 80 C, upon which crystalline Alpha Al(OH)3 forms directly. If, however, the reaction is below room temperature or performed too quickly,
bayerite is first formed which slowly converts into Alpha Al(OH)3.
I hope this helps.
[Edited on 7-9-2011 by AJKOER]
[Edited on 7-9-2011 by AJKOER]
|
|
|
Neil
National Hazard
   
Posts: 556
Registered: 19-3-2008
Member Is Offline
Mood: No Mood
|
|
You can grow tiny crystals by seeding a pool of aluminum hydroxide slime and waiting a long time - AJKOER seems to have that covered.
But if you are trying for sizable pieces try scrapping them off of garnet sand paper.
AFAIK there are no ways to grow saffire or ruby at home without a vacuum kiln.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by D4RR3N  | How can I grow an aluminium oxide crystal without the need of actually melting aluminium oxide (melts at over 2000C!)
What temperature would aluminium hydroxide decompose into aluminium oxide?
|
It maybe possible to dry Al(OH)3 (to Al2O3) by means of azeotropic distillation. I recall reading about that for drying Zr(OH)4 but can't recall the
azeotroping solvent that was used. At most that will yield micro-crystals, not macro-crystals.
I don't think growing larger crystals of annealed alumina is possible w/o prolonged high temperatures. If it was easier we'd all be producing
sapphires and rubies. Another GRQ scheme thwarted! 
|
|
|
D4RR3N
Hazard to Others
  
Posts: 271
Registered: 9-1-2007
Member Is Offline
Mood: No Mood
|
|
I was looking for larger crystals, Why would you need a vaccum furnace, it is an oxide?
|
|
|
Neil
National Hazard
   
Posts: 556
Registered: 19-3-2008
Member Is Offline
Mood: No Mood
|
|
I over stated sorry, an inert gas filled furnace works but you need to flush it out very well and that much gas costs $$$
You are way above the realm of NiCrome wire for melting Alumina - you need graphite elements. Any O2 left in the kiln when it is heated will start
burrowing through the elements and transporting carbon into the alumina.
Vacuum is just cheaper then massive amounts of inert gasses. What do you need these for? You can buy large pieces of factory made saph/ruby for a lot
less then you can build the furnace, heat controller and what not to do it.
I tried to crack that nut, it cracked me.
|
|
|
IrC
International Hazard
    
Posts: 2710
Registered: 7-3-2005
Location: Eureka
Member Is Offline
Mood: Discovering
|
|
The only method I know of starts with a large melted mass of Al2O3 (nonstop heat), a seed crystal, and a very slow moving rod which pulls the crystal
out of the mass as it grows. I have seen nice looking Ruby rods for lasers made this way, by adding some Chromium doping.
Edit: forgot - in a vacuum.
[Edited on 9-7-2011 by IrC]
"Science is the belief in the ignorance of the experts" Richard Feynman
|
|
|
unionised
International Hazard
    
Posts: 5128
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
This is probably the least implausible way to grow alumina crystals a home.
http://en.wikipedia.org/wiki/Verneuil_process
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
Actual at home a long time ago, my friend and I played with an Atomic Hydrogen flame by simply passing generated H2 through an electrical arc.
The flame temperature actually exceeds what is required for the production of synthetic gems. The chemical issue is providing a good sustainable
production source for H2 gas. My friend handled the electronics.
|
|
|
Neil
National Hazard
   
Posts: 556
Registered: 19-3-2008
Member Is Offline
Mood: No Mood
|
|
alumina is an electrical insulator...
|
|
|
unionised
International Hazard
    
Posts: 5128
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
So...?
|
|
|
Neil
National Hazard
   
Posts: 556
Registered: 19-3-2008
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by AJKOER  | ... played with an Atomic Hydrogen flame by simply passing generated H2 through an electrical arc.
The flame temperature actually exceeds what is required for the production of synthetic gems... |
@unionised;
So you can not use a hydrogen arc torch... 
|
|
|
D4RR3N
Hazard to Others
  
Posts: 271
Registered: 9-1-2007
Member Is Offline
Mood: No Mood
|
|
I have a vacuum pump and a cylinder of argon, I have a fused quartz tube, got some ceramic fibre somewhere too so half way there. Now these graphite
elements look interesting but how much would the controller cost? I don’t think the elements themselves are going to be that expensive but I bet the
controllers are!
|
|
|
Neil
National Hazard
   
Posts: 556
Registered: 19-3-2008
Member Is Offline
Mood: No Mood
|
|
1600-1725 °C wiki melting point range for Quartz
2072 °C - wiki melting point for Alumina
|
|
|
Panache
International Hazard
    
Posts: 1290
Registered: 18-10-2007
Member Is Offline
Mood: Instead of being my deliverance, she had a resemblance to a Kat named Frankenstein
|
|
decent tubes of al2o3 can be got from new or used sodium vapour lamps. When i kept melting the point oxygen feed on a glassblowing torch i was
playing with i replaced the stainless one with an alumina one from the lamp mentioned (the reason for my genius was that it happened to be sitting
directly in front of me on the bench). It is a reasonably easy material to shape, i was using a dremel, i expected it to be far more brittle than it
was. Anyway it glows bright on the torch when i use it in the way that i was, did the job.
If you know anyone who grows pot(or hydroponic tomatoes) ask them for an old lamp then jut cut off the glass outside or smash it and voila.
|
|
|
simba
Hazard to Others
  
Posts: 175
Registered: 20-5-2011
Member Is Offline
Mood: No Mood
|
|
Are rubies produced by the Verneuil Process as good as natural ones? Because the process seems yet to simple to me.
|
|
|
bobm4360
Hazard to Self
 
Posts: 60
Registered: 18-4-2011
Location: On a wretched little island.
Member Is Online
Mood: No Mood
|
|
Better! There aren't any inclusions. The process at lab scale is illustrated in Strong's "Procedures in Experimental Physics". This book is not
about physics experiments, but about building apparatus. There are chapters on glassblowing, high vacuum, high temperatures, and others. It's dated
(1938), but very readable and well illustrated.
Regards,
Bob
|
|
|
Paddywhacker
Hazard to Others
  
Posts: 478
Registered: 28-2-2009
Member Is Offline
Mood: No Mood
|
|
I have heard that small rubies can be produced as a side-product of the thermite reaction. You would need a thermite mix that didn't spray the
alumina out of the crucible, so ... a slow burning mix.
Maybe the addition of a flux could help the molten alumina to coalesce into larger blobs. Suitable fluxes could be determined experimentally, but
might include sodium chloride, sodium carbonate, ground glass, silica powder ...
|
|
|
unionised
International Hazard
    
Posts: 5128
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Neil  | Quote: Originally posted by AJKOER  | ... played with an Atomic Hydrogen flame by simply passing generated H2 through an electrical arc.
The flame temperature actually exceeds what is required for the production of synthetic gems... |
@unionised;
So you can not use a hydrogen arc torch...  |
Why not?
I can do soldering (at about 200C) with a blowtorch (about 2000C)
|
|
|
turd
National Hazard
   
Posts: 800
Registered: 5-3-2006
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by IrC  | | The only method I know of starts with a large melted mass of Al2O3 (nonstop heat), a seed crystal, and a very slow moving rod which pulls the crystal
out of the mass as it grows. I have seen nice looking Ruby rods for lasers made this way, by adding some Chromium doping. |
What IrC describes sounds more like Czochralski than Verneuil. If the second post was a response to the first, that is.
CVD is performed far below the melting point of alumina. Practicable in a home setting? I doubt it.
Edit: Hydrothermal is another possibility which might be the most accessible in a home setting. Though the conditions needed according to a quick
Google search are not encouraging (supercritical).
[Edited on 25-9-2011 by turd]
|
|
|
Neil
National Hazard
   
Posts: 556
Registered: 19-3-2008
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by unionised  | Quote: Originally posted by Neil  | Quote: Originally posted by AJKOER  | ... played with an Atomic Hydrogen flame by simply passing generated H2 through an electrical arc.
The flame temperature actually exceeds what is required for the production of synthetic gems... |
@unionised;
So you can not use a hydrogen arc torch...  |
Why not?
I can do soldering (at about 200C) with a blowtorch (about 2000C)
|
...because alumina is an insulator?
How are you going to get an arc to travel preferentially to a material that is not conductive? Nothing to do with temperature.
I recently talked to a fellow who made some small rubies in a electric furnace using this method
http://sciencelinks.jp/j-east/article/200601/000020060105A09...
And from below
http://pubs.acs.org/doi/abs/10.1021/ja049678v
"the crystal growth was conducted by heating a mixture of solute (Al2O3 + 0.5 mass% Cr2O3) and flux (MoO3) at 1100 °C, followed by holding the
solution at this temperature for 5 h."
1100°C it is much more in the range of DIY gear
@D4RR3N, Sounds like you might already have the stuff to do it.
|
|
|
Neil
National Hazard
   
Posts: 556
Registered: 19-3-2008
Member Is Offline
Mood: No Mood
|
|
A bee in my bonnet...
I used to have a small furnace with a roughly 5Al2O3/SiO2 refractory and Cr2O3 doping.
It had a ~2"x8" bore and mainly was run on propane, having been built to hold sawed off CO2 cartridges for micro melts. I used the same burner on it
that I used on a 6"X12" furnace, to point, it was very hot.
It ended up being primary used to sinter test pieces of ceramic, but not before it was contaminated with some borax flux and wood ash.
I still have the mini plinth block turned kiln table that I used for all of the furnaces life and curiously there is a layer of clear running to
pink/red glass smeared on one side. The smear is around five years old so I'm not positive I remember correctly but IIRC it was caused by borax
landing on the plinth however, it may also have been wood ash.
At any rate the smear was not sticky once it had been super heated during a crucible sintering attempt so I left it and never thought about it till
now.
I can not scratch it with a uncoated hacksaw blade or a bastard file. It scratched the carbide on a wood saw blade and seemed to be scratched by the
carbide but only very lightly.
The only data on the blade about the carbide is that the blade was "Manufactured from quality steels and carbides" I'd guess it would be C1 or C2?
Under a microscope at 100X it has no distinct crystals but does contain lots of bubbles.
It has taken heats of over 3000°F many times and is still around, it cuts glass with ease.
I have nothing with a known hardness to test it with 
There was obviously carbon in the furnace and the formation of true ruby is far to finicky IMHO to be formed by crude accident, which leaves me at a
loss to explain it.
Thoughts? Could it be crystalline alumina?
I only have a small sample so I'm loath to start boiling it in acids, thoughts on how to non-destructively identify it?
|
|
|
Wizzard
Hazard to Others
  
Posts: 337
Registered: 22-3-2010
Member Is Offline
Mood: No Mood
|
|
I think I found my next project 
MoS2 is converted to MoO3 in bake with oxygen rich environment, so I'll do that after drawing a vacuum after purging with an inert gas, and then the
MoO3 is water soluble- Excellent way to get it out of the mix  Then dry, and
dessicate, recrystallize and dessicate again. Or I suppose I could just purchase it. Then dry, and
dessicate, recrystallize and dessicate again. Or I suppose I could just purchase it.
Then, pop it all in a quartz tube, fire it up, and hope for some results! I'll likely boil out all the MoO3 into a cooler vessel. From there, analyze
results, and improve! I think my crystal lust is reaching a peak here, next I'll only want to make diamond!
|
|
|
peach
Bon Vivant
    
Posts: 1428
Registered: 14-11-2008
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Neil  |
...because alumina is an insulator?
How are you going to get an arc to travel preferentially to a material that is not conductive? Nothing to do with temperature.
|
The arc goes between two electrodes and through hydrogen, then the hydrogen transfers the heat to a none conductive surface;
| Quote: | Atomic hydrogen welding (AHW) is an arc welding process that uses an arc between two metal tungsten electrodes in a shielding atmosphere of hydrogen.
The process was invented by Irving Langmuir in the course of his studies of atomic hydrogen. The electric arc efficiently breaks up the hydrogen
molecules, which later recombine with tremendous release of heat, reaching temperatures from 3400 to 4000 °C. Without the arc, an oxyhydrogen torch
can only reach 2800 °C.[1] This is the third hottest flame after cyanogen at 4525 °C and dicyanoacetylene at 4987 °C. An acetylene torch merely
reaches 3300 °C. This device may be called an atomic hydrogen torch, nascent hydrogen torch or Langmuir torch. The process was also known as arc-atom
welding.
The heat produced by this torch is sufficient to melt and weld tungsten (3422 °C), the most refractory metal. The presence of hydrogen also
acts as a gas shield and protects metals from contamination by carbon, nitrogen, or oxygen, which can
severely damage the properties of many metals. It eliminates the need of flux for this purpose.
The arc is maintained independently of the workpiece or parts being welded. The hydrogen gas is normally diatomic (H2), but where the temperatures are
over 600 °C (1100 °F) near the arc, the hydrogen breaks down into its atomic form, simultaneously absorbing a large amount of heat from the arc.
When the hydrogen strikes a relatively cold surface (i.e., the weld zone), it recombines into its diatomic form and rapidly releases the stored heat.
|
The bigger problem there might be having an excess of atomic hydrogen present at thousands of degrees, since the goal is forming, or melting, oxides.
A Browns gas generator may be a better idea, as you'll get a balanced atmosphere out of that.
Hydrogen is not a great thing to have in a weld either, as that'll also embrittle the join. Which is part of the reason why GTAW (TIG) with Argon is
used now.
Quote: Originally posted by shivas  | | Are rubies produced by the Verneuil Process as good as natural ones? Because the process seems yet to simple to me. |
The equipment is certainly more basic than the CVD chambers, but I have a sneaking suspicion it may be fairly difficult to get a clean lump of ruby
out of it. It'll probably need at least a few goes to get it all tuned.
I expect the first few goes will result in a melted support and chamber or the ruby integrated into the support.
If you have a hydrogen cylinder or generator, give it a go. Take photos!
That'd make a wedding ring worth boasting about, home made gems and home refined precious metals.
Not so sure about this though, diamonds made from human ashes.
"Made it myself...", "At a jewellery class?", "From my dead cat..."
------------------------------------------------------------------
Irving Langmuir

Atomic hydrogen torch, this article has details of the current, voltage, electrode sizes and further design elements of the device.

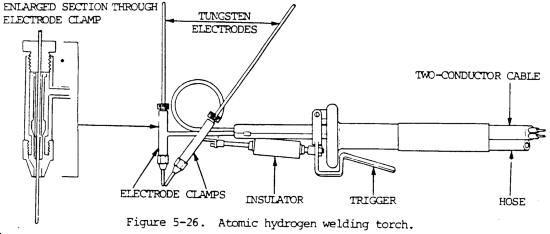
Buy two of these

And a roll of that

A high tech atomic hydrogen source

You can see a Czochralski crystal furnace in operation towards the start of this video;
<iframe sandbox width="640" height="480" src="http://www.youtube-nocookie.com/embed/aWVywhzuHnQ" frameborder="0" allowfullscreen></iframe>
[Edited on 30-9-2011 by peach]
|
|
|
Neil
National Hazard
   
Posts: 556
Registered: 19-3-2008
Member Is Offline
Mood: No Mood
|
|
Thank you Peach, sorry Unionised; I was under the incorrect impression it was more like a heli-arc set up.
Browns gas may indeed be wonderful for melting alumina, its oxidizing flame may be useful in the material prep as well as the actual melting. Only
most Browns gas torches I've seen people build have a high rate of self destruction... 
|
|
|
| Pages:
1
2
3 |