Retard-3000
Hazard to Self
 
Posts: 55
Registered: 17-11-2010
Member Is Offline
Mood: No Mood
|
|
Nitroaromatic Reduction w/Sn
Does anyone have a diagram or know how Nitroaromatic reductions using Tin occur/proceed, such as the reduction of Nitrobenzene ?
I understand that the Tin reacts with the Hydrochloric acid producing Tin (II) Chloride and Hydrogen gas, then the Tin (II) Chloride reduces the nitro
group whilst subsequently being oxidised to Tin (IV) Chloride. I'm not sure how it occurs exactly though, does anyone know how the reaction would
proceed step by step?
|
|
|
redox
Hazard to Others
  
Posts: 268
Registered: 22-2-2011
Location: The Land of Milk and Honey
Member Is Offline
Mood: Chalcogenetic
|
|
Here is commonly accepted mechanism. The tin is the source of electrons, and the Hydrochloric acid is the source of hydronium ions. The methanol is
the solvent and source of H atoms.
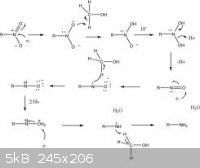
EDIT: That picture showed up a little small, I don't know how to make it bigger.
[Edited on 14-8-2011 by redox]
My quite small but growing Youtube Channel: http://www.youtube.com/user/RealChemLabs
Newest video: Synthesis of Chloroform
The difference between chemists and chemical engineers: Chemists use test tubes, chemical engineers use buckets. |
|
|
Retard-3000
Hazard to Self
 
Posts: 55
Registered: 17-11-2010
Member Is Offline
Mood: No Mood
|
|
Thank you so much  , I was able to find a larger image of the picture on google
by searching for the name of the image , I was able to find a larger image of the picture on google
by searching for the name of the image 
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Since when is that a "commonly accepted mechanism"? Where is the reference? Why would the intermediate radicals abstract alpha-hydrogens from methanol
when they can just receive another easy to get electron from the metal surface? And why would the mechanism involve alcohols when the reduction works
just fine in their absence?
What is wrong with the general metal dissolving reaction mechanism for nitrobenzene to aniline reduction as presented in March's (chapter
19-45 Reduction of Nitro Compounds to Amines)? At least it involves no hydrogen abstraction, only simple electron and proton
transfers, just as one would intuitively expect. The 6th edition has a nice scheme (page 1818) for the proposed mechanism and gives this reference:
H.O. House, Modern Synthetic Reactions, 2nd ed., W.A. Benjamin, NY, 1972, p. 211. I suppose that is not the original reference, so those
interested will have to hunt down the reference to the primary literature to get an answer.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
redox
Hazard to Others
  
Posts: 268
Registered: 22-2-2011
Location: The Land of Milk and Honey
Member Is Offline
Mood: Chalcogenetic
|
|
I am currently on vacation, when I get back home I'll look at my copy of March. I'm sorry, I just googled the mechanism and found that picture. My
sincerest apologies Nicodem if this is not the correct mechanism.
My quite small but growing Youtube Channel: http://www.youtube.com/user/RealChemLabs
Newest video: Synthesis of Chloroform
The difference between chemists and chemical engineers: Chemists use test tubes, chemical engineers use buckets. |
|
|
Davyrocket
Harmless

Posts: 20
Registered: 10-5-2013
Member Is Offline
Mood: Euphoric if it works
|
|
Hi all in scieance madness....I know this is a old thread...but
However I too am intreasted in the Reduction of SnCl
With nitro compounds.
I have read about lead free solder wich contains a fairly high % of sn
But there would still be a lot of impurity,and would assume this would
Have a yield effect on final product.
I have also read some where that SnCl it is superior to al/hg reduction
As nitro compounds go and due to the high toxcity of the al/hg
This would be a much better experiment for me to use with my new glassware
I recived for my birthday
Also has any one experienced the two diffrent reactions.
I am not in no means looking for spoon feeding, I am willing to study what ever method is best I know what rout to try.
ie...is there a yield difference between them...is there a scale difference
Is ther much difference on work ups....
thx the rocket
|
|
|