| Pages:
1
2 |
daydreamer
Harmless

Posts: 7
Registered: 21-11-2003
Member Is Offline
Mood: No Mood
|
|
methyl iodide
Generally speaking couldn't most reactions that call for dimethylsulfate be substituted with methyl iodide. I am wanting to attemt reactions that
call for this material but would rather not use dimethylsulfate because of the risk involved.
|
|
|
Quantum
Hazard to Others
  
Posts: 300
Registered: 2-12-2003
Location: Nowhereville
Member Is Offline
Mood: Interested
|
|
I don't know much about dimethylsulfate but I guess the answer varies with what sort of reactions you have in mind.
Better ask a smarter member.
What if, what is isn\'t true?
|
|
|
BromicAcid
International Hazard
    
Posts: 3245
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Well, since this is in the beginnings section I think I'll go a little in depth on the quick down and dirty thought process to determine on a
preliminary level if two chemicals are able to be swapped.
First off you would need to see if they are compatible on just the physical level.
Both are liquids and you would want to compare their solubilities to see if they are similar. Going to a handy dandy chemical encyclopedia you can
find
Methyl Iodide: Soluble in alcohol and ether, partially soluble in water.
Dimethyl Sulfate: Soluble in alcohol, ether, and water.
They sound similar so far, they should behave similarly on a physical level under normal standard-state reaction conditions. Also when you're
thumbing though your book compare other things like BP and MP, the BP of methyl iodide is much lower then DMS so this will affect reactions at
slightly elevated temperatures. Also you might want to compare other properties, websites like this one are helpful for that, dipole moment, viscosity, the closer they are the better.
Similar on a physical level, that's about 1/5 of it. The chemical level though is a whole 'nother story. They are both methylating agents,
that;'s a start, but what else? Does one possess additional properties? Dimethyl Sulfate is also a dehydrating agent to some degree something
that methyl iodide is not, but that's minor. Look for obvious differences, like is one a powerful oxidizer and the other not, one might reduce
and the other be able to be easily reduced, each of these properties might be in addition to other properties that might have inclined you to chose
this chemical as a substitution in the first place. The easiest way to check this online is to just run a google and see if you can find identical
reactions run with each.
As luck would have it there are a number of reactions that can be found by typing in something like "dimethyl sulfate methyl iodide properties
organic chemistry" that show the two are somewhat interchangeable. But this really is where you have to do your homework. You have to compare
reaction conditions over multiple reactions in comparison to one another. You also need to look at the structures, e.g. dimethyl sulfate has two
methyl groups, after one has gone to methylating something is the other active enough to methylate further? If not then it would be interchangeable
on a mol for mol basis with MeI. But mostly it's experience and testing.
Even if their physical properties were not the same there are always phase transfer catalysts and vigorous stirring to help it along. However in this
specific instance I believe that DMS is interchangeable with Methyl Iodide with only slight modifications to most reactions, save those run at
temperatures above 40C where of course the methyl iodide would begin to evaporate.
[Edited on 3/7/2004 by BromicAcid]
|
|
|
daydreamer
Harmless

Posts: 7
Registered: 21-11-2003
Member Is Offline
Mood: No Mood
|
|
Thank you that is must helpful. I am young and beginning my amateur hobbie.
Thank you for your patience
|
|
|
tryptamine
Harmless

Posts: 16
Registered: 20-3-2003
Member Is Offline
Mood: elated in love
|
|
hold on a minute
I reccomend looking at using whatever is convenient with the proper precautions. Methyliodide is at least as if not more dangerous than DMS.
|
|
|
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by tryptamine
I reccomend looking at using whatever is convenient with the proper precautions. Methyliodide is at least as if not more dangerous than DMS.
|
Very interesting!
I have made methyl iodide and always treated it with great care, but never found reliable information about it´s dangers!
I have been planning to do it again and would be grateful if anyone could post links and anecdotal information about its dangers.
|
|
|
ziqquratu
Hazard to Others
  
Posts: 385
Registered: 15-11-2002
Member Is Offline
Mood: No Mood
|
|
For a start, it's a potent methylating agent, meaning that it is going to be highly carcinogenic. It's also readily absorbed through the
skin, making it even worse - resistant gloves are an absolute must. Not only that, MeI is somewhat volatile (BP 42-43*C), certainly more so than
other methylating agents like DMS, meaning that there's a good chance that you'll be breathing great lungfulls of the stuff if you're
not careful. It causes severe skin blistering, can cross the placenta if someone happens to be pregnant, and if carcinogenicity wasnt enough it
hydrolyses to methane and hydriodic acid (HI), burning the crap out of whatever body region it happens to got to. Overall, it's really not a
happy substance to just go nuts with.
Have a look at the MSDS for MeI - it'll tell you most of the things that you'll want to know about it. In fact, that's pretty good
all-round advice - when planning to play with a chemical you know nothing about, have a look at the MSDS first!
|
|
|
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by ziqquratu
(snip)
Have a look at the MSDS for MeI - it'll tell you most of the things that you'll want to know about it. In fact, that's pretty good
all-round advice - when planning to play with a chemical you know nothing about, have a look at the MSDS first! |
Thank you ziqquaratu,
but I'm afraid that's exactly the point: Take a look at these informations about methyl iodide:
http://www.intox.org/databank/documents/chemical/methylio/ci...
http://www.intox.org/databank/documents/chemical/methylio/ia...
Basically they say there is no evidence of carcinogenicity on humans, which I find hard to believe, and you would need a lot of exposure to suffer
damage. It also says the oceans put a lot of it in the air.
I believe methyl iodide is a dangerous chemical, but those pages are not as scary as I thought they would be. Any text on DMS screams its horrible
nature.
Thats why asked about ANECDOCTAL information. People who worked with it or some text on the net.
|
|
|
PainKilla
Hazard to Others
  
Posts: 306
Registered: 29-4-2004
Member Is Offline
Mood: No Mood
|
|
| Quote: |
I have produced METHYL iodide a few times. The yield varies wildly with small changes in the reactant ratios and reaction conditions. The best yield I
ever got was mixing 25gKI (0,15mol), 25ml 85% Phosphoric acid (0,37 mol), 25ml of methanol (0,62mol) and 0,1g of iodine crystals. This was brought to
gentle reflux for 1 hour using ice-cold water in the condenser and magnetic stirring, then the reaction was left for 24 hours in a closed flask.
Finally it was fractionally distilled (with magnetic stirring!) to give 6ml of MeI (13,7g, 0,096mol) a 64% yield.
You add some water to the distilate to obtain two phases, the lower is the very volatile iodide.
Would the yield be lower without the iodine crystals? I don’t know.
Does it have to be refluxed a bit and left for 24 hours? In my experience, yes. Be my guest to try something different.
For fractional distillation I used two condensers in series, one going “up” with water at 22°C (ambient), and the other going down, with ice-cold
water. The receiving flask was kept in a ice-water mix.
|
I plan on making some methyl iodide too, probably following this procedure. I don't quite understand however what is meant by the fractional
distillation flask setup you have. Could one not simply do as you said, bu redistill the end product... it would be much easier I think, though, I am
not sure whether it would work or not.
Also, doesn't adding water make the MeI go away, reacting into HI and MeOH?
|
|
|
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by PainKilla
I plan on making some methyl iodide too, probably following this procedure. I don't quite understand however what is meant by the fractional
distillation flask setup you have. Could one not simply do as you said, bu redistill the end product... it would be much easier I think, though, I am
not sure whether it would work or not.
|
I have done this reaction a few more times and learned a lot.
1- It's not necessary to add iodine crystals.
2- It's not necessary to reflux and leave overnight.
3- The reaction should be quite hot. That is: strong reflux of the methanol.
4- The whole point of the reaction is to make the methanol go back to the reaction (reflux it vigorously) but alow the formed methyl iodide to
"escape" to an ice-cold condenser.
The idea is to keep methanol reacting as long as possible.
Since the BP of MI is about 42ºC and the BP of Methanol is some 20ºC above that, the way to do it is something like the attached picture.
Control the flow of tap water to make sure the methanol condenser stays warm and let some vapours pass to the cold consensator. Otherwise nothing
distills.
The reaction should take about 30 minutes, and the distilate should be something about 20 ml to yield, after addition of water, 6ml of MI.
As the reaction proceeds, a lot of the methanol goes to the receiving flask but, when you add water, methyl iodide forms a brown layer in the bottom.
Some NaOH in the water and some shaking make it more clear (not brown) by removing the iodine.
| Quote: |
Also, doesn't adding water make the MeI go away, reacting into HI and MeOH? |
No, it seems to be very insoluble and inert relative to water. I keep it for weeks with a layer of water on top to prevent evaporation. On a closed
flask of course.
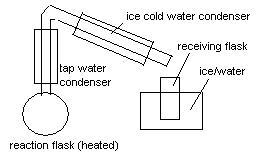
|
|
|
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
I'm afraid that editing will remove my attachment so I'm making a new post:
Notes- 1 - my tap water is almost always above 20ºC, maybe yours isn't.
2- I have always been very sucessfull with my methylenations of phenols using MI, NaOH and DMF (dimethyl formamide). After the reaction is complete,
just add water and filter the precipitate. Edit: sometimes you can steam-distill your product directly.
3- I still have no trustworthy information on MI (see posts above), so, I'm very carefull with it.
[Edited on 4-5-2005 by Tacho]
|
|
|
Kalle anka
Harmless

Posts: 15
Registered: 10-6-2005
Location: Scandinavia
Member Is Offline
Mood: decomposing
|
|
Cannot the relatively low yields (64%) be due to loss by the volatility of MeI? From my books, making an alkyl iodide from alcohol and hydrogen
halides should be nearly quantitative.
Have anyone tried to make methyl iodide by refluxing and destilling of from a stoichiometric combination of HCl, KI and CH3OH ? I would want try this
but have no methanol at this time..
Concerning the toxicity of methyl iodide, you can find its msds by simply googling for "methyl iodide msds pdf" or similar. I would rather
use methyl iodide than methyl sulfate for my methylations. There is some chemicals that i just dont dare to "touch" and DMS is one of them
EDIT: OMFG! I've read the papers included above:
Skin Contact:
A pad soaked in methyl iodide and held against the forearm for 10 minutes produced stinging and reddening;
What the hell would happen if you did that with DMS!? Death..
[Edited on 14-6-2005 by Kalle anka]
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
| Quote: |
From my books, making an alkyl iodide from alcohol and hydrogen halides should be nearly quantitative.
|
Tertiary alcohols, yes. Secondary, maybe. Primary, no.
64% is a pretty good yield anyway.
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
Kalle anka
Harmless

Posts: 15
Registered: 10-6-2005
Location: Scandinavia
Member Is Offline
Mood: decomposing
|
|
| Quote: | Originally posted by vulture
Tertiary alcohols, yes. Secondary, maybe. Primary, no.
|
Notice i was saying alkyl IODIDES, you are talking about reacting a tertiary alcohol with hydrogen chloride which proceeds so slowly with secondary
and primary alcohols that a lewis base is needed.
|
|
|
Sandmeyer
National Hazard
   
Posts: 784
Registered: 9-1-2005
Location: Internet
Member Is Offline
Mood: abbastanza bene
|
|
Yes, it works great, it gives 80% yield, at least when done with sulfuric acid. It is however messy due to oxidation to I2. I've seen in the
litterature phosphoric being used, but only are the yields good when 2 ekvivalents KI and the acid of 95% strength is used.
For higher homologs, better make alkyl bromides, it is both cheaper and has cleaner workup. Then for the alkylations use alkyl bromides with DMF or
NMP. Thruout Pihkal acetone is used, but these solvents leave acetone in the dust. Anyways, mix 0.2 mol NaBr with 25 mL water followed by 0.18 mol
alcohol while stirring. Cool on ice and slowly add 0.37 mol H2SO4 (d=1.84). Reflux for 30 min, allow to cool and distill the alkylbromide. Wash it
with 25 mL water followed by 15 mL cold H2SO4 to remove the alcohol that might have come along under dist. Separate, this time keep upper layer (H2SO4
d=1.84) and wash it with 15 mL 10 % NaOH. Dry the organic phase over MgSO4 and distill once again.
[Edited on 16-6-2005 by Sandmeyer]
|
|
|
Kalle anka
Harmless

Posts: 15
Registered: 10-6-2005
Location: Scandinavia
Member Is Offline
Mood: decomposing
|
|
I made methyl iodide myself today.
First i heated 50grams of 85% phosphoric acid at a gas flame. It gives off some water and then stabilizes. I let the flask cool to below 35 deg C and
added 35.600 grams of sodium iodide, and added some 50ml methyl alcohol (extreme excess) all at once. I put up a simple distillation setup and heated
the mixture at the gas flame. As Tacho did, I poured some water, probably 5ml in the reciever flask and soon methyl iodide came over and sank to the
bottom, whebn methyl alcohol became apparent i closed the flame and let the flask cool. Then I removed the methyl iodide with a pipette and addded
this to a tared brown flask with 2ml water and some copper shavings. I put back the methyl alcohol and water mixture in the reaction flask and added
some 50ml phosphoric acid and reheated it; again some methyl iodide collected under the water layer as before and was again collected, i repeated this
procedure another time but very little methyl iodide came over this time, most methyl alcohol. To this i added some cool water saturated with salt and
the mixture became turbid and some methyl iodide settled to the bottom of the flask and became a droplet. I collected this droplet and measured the
flask, 21.140 grams of methyl iodide.
That is 63% of theory.. Tacho's experience was confirmed. I did not use an ice bath and his "rising condensor reflux aid", and i used
some 15 degrees cool water for my liebig condensor.
Beware that methyl iodide has a significant vapor pressure and some skill is needed to collect it with the pipette; i found that transferring
methylene chloride has helped gain that skill, but be careful with this stuff as it is treated as a poison..
It is also interesting that some iodide is oxidized and gets liberated from the sodium iodide in the reaction flask and colors the mixture brown.
|
|
|
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
Congratulations Kalle! Interesting aproach to the problem. I would think the water introduced would lower the yield, but 63% is as good as I ever got.
Why copper shavings?
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
Nice work! 
| Quote: |
It is also interesting that some iodide is oxidized and gets liberated from the sodium iodide in the reaction flask and colors the mixture brown
|
Odd that this happens, but I suppose that's the reason why you add the copper shavings?
Also, how could you tell the MeOH was coming over?
What are you planning to do with the MeI?
[Edited on 9-8-2005 by chemoleo]
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
neutrino
International Hazard
    
Posts: 1583
Registered: 20-8-2004
Location: USA
Member Is Offline
Mood: oscillating
|
|
| Quote: | | It is also interesting that some iodide is oxidized and gets liberated from the sodium iodide in the reaction flask and colors the mixture brown.
|
NaI does this by itself. A simple solution of the stuff shows that brown color for some reason.
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
O2 dissolved in the water maybe?
Tapwater around here makes a mess when added to lead acetate solution (due to carbonate and/or sulfate ions) and strong reducers (good luck getting
only ferrous hydroxide after washing).
Tim
|
|
|
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
Yes, the solution turns brown. It has nothing to do with water, no water is added to the flask. I guess the dinamic of the reaction has some free I2
in equilibrium.
By the end of the distillation , some purple fumes evolve. At this point, no more MeI is made.
The MeI is also brown due to I2. I wash it with dilute NaOH solution to make it colorless.
Why copper shavings?
|
|
|
Sandmeyer
National Hazard
   
Posts: 784
Registered: 9-1-2005
Location: Internet
Member Is Offline
Mood: abbastanza bene
|
|
Here is a mild and simple method for sec, tert and benzyl iodides. It dosen't affect primary.
Direct Conversion of Alcohols into the Corresponding Iodides
Reni Joseph, Pradeep S.Pallan, A.Sudalai and T.Ravindranathan
Tetrahedron Letters, Vol. 36, No. 4, pp. 609-612, 1995
Abstract: A mild and effective procedure for directly converting secondary, tertiary and benzylic alcohols into the corresponding
iodides is described using I2 in refluxing petroleum ether. The reaction proceeds with inversion of configuration.
Experimental: In a typical experiment, cycloheptanol (0.751 g; 5 mmol) was added to the stirred solution of iodine (1.269 g; 5 mmol)
in dry petroleum ether 60-80 C (25 ml) and the reaction mixture was refluxed for 1 h. The progress of the reaction was monitored by TLC (petroleum
ether 60-80"C as the solvent). After the reaction was complete, the contents were cooled and diluted with petroleum ether (25 ml). It was then
washed successively with 10% aqueous thiosulphate, water and brine. Drying (anhy. Na2SO4) and evaporation of the solvent afforded the crude product
which was purified by column chromatography (silica gel : pet ether) to give cycloheptyl iodide (0.84 g; 75%).
[Edited on 9-8-2005 by Sandmeyer]
|
|
|
Kalle anka
Harmless

Posts: 15
Registered: 10-6-2005
Location: Scandinavia
Member Is Offline
Mood: decomposing
|
|
Nice read Sandmeyer!
I added copper shavings to stabilize the methyl iodide in the storing flask (not in the reaction flask), it is common to do this, and i believe that
you even can read this in some MSDS sheets of methyl iodide..
I saw that MeOH was coming over when the drops that fell into the water in the reciever didn't fall to bottom, but rather dissolved into the
water layer 
I did not see the purple iodine vapors that you saw, Tacho, but i noticed that the liquids condensing on the top of the distillation adapter (that
with the thermometer sticking out on top of it, and condesor to the side of it) and the thermometer got brownish; i immediately stopped the heating
when i saw this, but inevitably some fell to the reciever and colored the water here brownish
Actually the MeI did not get a brownish color, not even a slight tint; it was rather a milky-ish liquid which i just collected and added to the
storing flask, where it got completely clear.
BTW, the reason why i added excess phosphoric acid and MeOH was an attempt to push the existing equilibrium very to the MeI side, but i'll try to
increase these excesses next time; Also i saw today that yesterdays brown solution which i saved (ACS grade NaI is pretty expensive) turned very
brown, almost like the "iodine tincture" you get at pharmacy for use as an antiseptic; probably auto-oxidation..
I am using it for alkylations (of course), se another, new thread about this: "crap yields of phenol-alkyl ether"
|
|
|
armo
Harmless

Posts: 11
Registered: 23-8-2005
Location: Brasil
Member Is Offline
Mood: Cool....
|
|
KI darkening
Hi,
I think KI just oxidate in presence of light. That is an old problem for people that deals with ozone titration.

|
|
|
einstein(not)
Hazard to Self
 
Posts: 50
Registered: 14-12-2006
Member Is Offline
Mood: No Mood
|
|
Just completed second attempt at MeI with unexpected results. Reaction was as follows:
30 gr. moist I (tincture)
5 gr. rp (matchbooks)
75 ml. methanol
Were placed in 500 ml RBF with a short air cooled condenser connected vertically to which was connected a long water cooled condenser set for downward
distillation to a 250 ml flask in which was 10 ml of distilled water. RBF was heated until the temp. at the top of the air cooled condenser was at
55C and held there for 4 hours. Approx. 15 ml of MeI came over and then nothing else. Heat was discontinued (bedtime) for 12 hours then restarted
again at same temp for 2 hours. Approx. 5 ml more of MeI came over and the water cooled condenser was emptied into the receiving flask. The MeI
was collected and placed in a small amber bottle.
The remaining water/methanol was returned to the RBF and heated as before except this time to 65C for 4 hours. Approx. 40 ml of amber colored
methanol (or what I assume was methanol) came over. The receiver was changed and the tempature increased to maximum. At approx. 80C a white vapor
began to appear which condensed to a milky white watery liquid in the receiver. I had a vacuum adapter attached to the top of the receiver with a
hose attached and led into a flask of NaOH solution for venting purposes. I tested the gas that bubled out a few times and it was flamable burning
with alot of soot. Very little of this gas was produced. The water cooled condenser had small amounts of white sediments in the bulbs which had
separated out. Upon disconnecting it to wash down these sediments I noticed a slight amount of ignition at the joint. And closed the system
quickly. I then turned out the lights and slightly lifted the stopper in one neck of the flask. It sparked and glowed quite a bit and I lowered it
back into place. I then removed the thermometer adapter and wash distilled water down both condensers and left everything to cool.
The next day I turned out the lights again and checked the stopper. Still some sparks and glowing but not as much as before. I added more water
quickly dismantled everything stoppering the flask quickly after removing the condenser from it. Upon removing the RBF from the mantle (ebay special)
I found it cracked and sagging. The bowl of the mantel has insulation missing and an exposed element so this didn't surprise me. Although it had
never cracked a flask before and this was a chemglas flask at that. I swirled the contents of the flask to wash down anything on the sides and pour
it all into a container. The inside of the flask had brown fluffly looking sediment in the bottom and a few pen point droplets scattered around the
top that where amber colored with red borders. I assume this to be phosphorus.
I broke up the flask and placed the peices into a beaker covered with water. Smokey vapors rose from the beaker for at least an hour even though
all the glass was covered with water. The milky liquid has the same vapors if slightly heated or exposed to UV light. There is a waxy looking glob
floating slightly above the bottom of the the flask the size of a pencil eraser. There appears to be no sediment other than this. I added a small
amount of ether to a sample and the ether took on an amber color.
My question is what is this milky liquid and does it have any value?
|
|
|
| Pages:
1
2 |