| Pages:
1
2 |
kuro96inlaila
Hazard to Self
 
Posts: 96
Registered: 21-6-2010
Location: Malaysia
Member Is Offline
Mood: Quietly thinking
|
|
Extraction of platinum metal from Sperrylite (PtAs2)
Few month ago I recieved few grams of sperrylite,from my uncle who is geologist.Sperrylite,which is an ore comprising Platinum and Arsenic as PtAs2.

Here is the picture of among the ores he gave me.
I want to extract platinum metal from this ore,which then I can use it to explore platinum chemistry.
But I am confused on how to extract the platinum metal from this ore safely,which arsenic is also present?
Burning this ore in big amount can be disaster.Releasing arsenic oxide fumes.And I've searched the internet there was no other info on sperrylite ore
other than its geological info.If you all have any text on this compound,can we share it here?
I have thought some way to extract it:
1)By passing hydrogen gas produced by zinc and hydrochloric acid reaction which then dried to heated platinum arsenide,making platinum metal and
arsine.
PtAs2+3H2--->Pt+AsH3
I plan to bubble the arsine in hydrochloric acid to yield arsenic trichloride solution.
Another way was to burn the gas produced and glass plate is placed in the flame to condense arsenic.Which is similar to Marsh's test.
But the I realised that arsine will start to decompose rapidly in 230C ,which I thougt reachable when platinum arsenide is heated.This would make
arsenic produced back in the reaction vessel.
This way isn't reliable,it will not guarantee the positive result and who wants to "play" with among the worst poisonous gas - arsine?
2)Platinum arsenide ore is attacked in both HNO3 and aqua regia producing nitrogen dioxide gas.
With HNO3 it produce lemon tinged solution,while in aqua regia it produce orange coloured solution.
I don't know the product in this solution and how to extract platinum from both solution.
Below are the question I asked in short question thread 3,which was not replied since:
Quote: Originally posted by kuro96inlaila  | These make me confuse:
Yesterday,I decided to conduct a couple of experiments with my sperrylite (PtAs2) mineral.
1.I put about half a gram of PtAs2 in nitric acid.Then I heat it.Sperrylite react with warm concentrated nitric acid producing a yellow solution.Then
I add few milimeter of ammonium chloride solution,hoping that ammonium hexachloroplatinate will be precipitated.But nothing happen.
I guess ammonium hexachloroplatinate only will be precipitated if ammonium compounds solution is added to chloroplatinic acid.While I might have
platinum nitrate in the yellow solution.So,I add magnesium ribbon,hoping that platinum metal precipitated out.It first bubbled,then a brownish orange
compound start to precipitated.
I can't figure what compound it is,so I set it aside and conduct other experiment:
2.I react PtAs2 with aqua regia,then I add ammonium chloride.But no ammonium hexachloroplatinate precipitated.Then I add sodium bicarbonate to the
solution.It first bubbled,releasing carbon dioxide.Then a brownish orange compound start to precipitate.The colour is similar to what was precipitated
in the other experiment.
So,what is these thing actually?I guess it would be either platinum dioxide monohydrate or sodium platinum chloride.But I also doubt it is an arsenic
compound.
And,if this is platinum's compound,roasting it should turn it to platinum metal right? |
here is the first experiment pic:

And for the second experiment:
And I heated the orange compound produced in the second experiment,its color stay unchanged.This lead me nowhere.
So I was confused on how to extract the platinum metal from this ore.Worst to come,how can I make it while disposing the arsenic safely?
Is there any method we can share here?
(Here is some text which may useful):
Attachment: Disposal of arsenic soluble compounds.pdf (289kB)
This file has been downloaded 877 times
|
|
|
kuro96inlaila
Hazard to Self
 
Posts: 96
Registered: 21-6-2010
Location: Malaysia
Member Is Offline
Mood: Quietly thinking
|
|
Any idea,anyone?
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
First, Google "disposal
arsenic insoluble". Next, find paper Arsenic Disposal Practices in the Metallurgical Industry. Third, read abstract and see "scorodite, FeAsO4.2H2O".
|
|
|
Wizzard
Hazard to Others
  
Posts: 337
Registered: 22-3-2010
Member Is Offline
Mood: No Mood
|
|
Maybe not the most efficient way, but try superheating it in a vacuum, and distilling out the arsenic? It boils at a reasonable furnace temperature.
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
The best way would be to find an analytical procedure that uses something like aqua regia to dissolve all the metal content of the ore and then
precipitates the platinum as say ammonium hexachloroplatinate.
A bit of book work could save you a lot of pain!
|
|
|
Fleaker
International Hazard
    
Posts: 1252
Registered: 19-6-2005
Member Is Offline
Mood: nucleophilic
|
|
Ehh, the best thing to do is leave it alone. Sperrylite in small quantities is worth FAR more as a geological specimen than for the platinum content.
It's a very rare mineral.
The conventional way to do this is to pulverise it and then roast in a stream of oxygen: the arsenic will distill as its volatile trioxide As2O3
(As4O6 dimer when distilling).
If you have to ask questions on how to do this, I really think you should not be handling arsenic at all, especially arsine gas.
Does your Uncle have much sperrylite? To the right people, it's worth quite a bit...
Neither flask nor beaker.
"Kid, you don't even know just what you don't know. "
--The Dark Lord Sauron
|
|
|
arsphenamine
Hazard to Others
  
Posts: 236
Registered: 12-8-2010
Location: I smell horses, Maryland, USA
Member Is Offline
Mood: No Mood
|
|
Become familiar with meso-dimercaptosuccinic acid.
|
|
|
kuro96inlaila
Hazard to Self
 
Posts: 96
Registered: 21-6-2010
Location: Malaysia
Member Is Offline
Mood: Quietly thinking
|
|
@watson.fawkes
Thanks!
@sciencesquirrel
I'll try that and I'll tell the result soon.
@wizard
Yes,I've think that way too.But I forgot to include it in the first post.But currently I dont have any vacuum pump and cold finger apparatus.
@Fleaker
How about channeling the arsenic oxides into hydrochloric acid?so the arsenic oxides did not escapes to the atmosphere.
Well,I did not see myself but,he tell me that if I can find a way to extract platinum from it,he will give his 4kg of sperrylite.
@arsphenamine
That compound is used to chelate platinum ions right?
[Edited on 21-4-2011 by kuro96inlaila]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by kuro96inlaila  | Well,I did not see myself but,he tell me that if I can find a way to extract platinum from it,he will give his 4kg of sperrylite.
|
Even if you succeed in extracting small amounts of Pt from a small sample, you’d probably kill yourself/seriously poison yourself treating the 4 kg
(choose your poison: arsine or arsenic trioxide!)
Tell your uncle that if he loves you he should give you the 4 kg on the proviso that you sell the stuff in small amounts using the ‘right
channels’ and that you share the huge windfall with him. Alternatively have him send the stuff to moi, I’ll be eternally grateful! 
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
Nice sperrylite is worth a fortune.
http://cgi.ebay.co.uk/HUGE-11MM-SPERRYLITE-CRYSTAL-NORILSK-R...
The 4kg must be worth thousands of dollars even if it is not very good.
The extraction is going to produce huge amounts of highly toxic waste containing large amounts of arsenic.
What are you going to do with that?
|
|
|
kuro96inlaila
Hazard to Self
 
Posts: 96
Registered: 21-6-2010
Location: Malaysia
Member Is Offline
Mood: Quietly thinking
|
|
Quote: Originally posted by blogfast25  | Quote: Originally posted by kuro96inlaila  | Well,I did not see myself but,he tell me that if I can find a way to extract platinum from it,he will give his 4kg of sperrylite.
|
Even if you succeed in extracting small amounts of Pt from a small sample, you’d probably kill yourself/seriously poison yourself treating the 4 kg
(choose your poison: arsine or arsenic trioxide!)
Tell your uncle that if he loves you he should give you the 4 kg on the proviso that you sell the stuff in small amounts using the ‘right
channels’ and that you share the huge windfall with him. Alternatively have him send the stuff to moi, I’ll be eternally grateful! 
|
Well,that's why I want to discuss this thing with sciencemadness comunity,to get the most reliable and safe way to extract this metal.
@sciencesquirrel
Hm,I plan to save beautiful sample among those 4kg.I do not want to process all of the mineral.
Well,for the toxic waste,I plan to use the instruction in the patent I include in my first post.Just I don't really understand the method,the way they
write the patent give me a headache.I need some explanation on that.
Or another way I will sent them to Enviromental department or waste processing centre to be disposed.
|
|
|
Fleaker
International Hazard
    
Posts: 1252
Registered: 19-6-2005
Member Is Offline
Mood: nucleophilic
|
|
Are you in North America? I know multiple parties that would be interested in buying.
Neither flask nor beaker.
"Kid, you don't even know just what you don't know. "
--The Dark Lord Sauron
|
|
|
kuro96inlaila
Hazard to Self
 
Posts: 96
Registered: 21-6-2010
Location: Malaysia
Member Is Offline
Mood: Quietly thinking
|
|
No,I did not live there.I live in Malaysia.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Someone should calculate how many people you could outright kill with the arsenic from 4 kg of that stuff  ! !
Definitely a case of a 'poisoned chalice'!
[Edited on 22-4-2011 by blogfast25]
|
|
|
unionised
International Hazard
    
Posts: 5126
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
About 10,000 people give or take a bit.
However it would be difficult t distribute the As evenly among them.
I think the best thing to do with the stuff would be to sell it in small quantities to collectors. But ,assuming some of it is not going to sell, then
smelting for Pt is not a bad option. You need to do something with the As- trapping it as the oxide or as sodium arsenate would look like the best bet
but, unless you are really sure what you are doing, I think you should sell it to someone who does.
|
|
|
plante1999
International Hazard
    
Posts: 1936
Registered: 27-12-2010
Member Is Offline
Mood: Mad as a hatter
|
|
i think leaching with sodium sulfite and sodium hydroxide mix and evaporate the leach to get sodium arsenate crystals.
pretty good idee to be acused of terorism act.....killing 6000 people.
[Edited on 22-4-2011 by plante1999]
I never asked for this.
|
|
|
kuro96inlaila
Hazard to Self
 
Posts: 96
Registered: 21-6-2010
Location: Malaysia
Member Is Offline
Mood: Quietly thinking
|
|
I think I determined to extract the platinum metal from this ore,I have think this since last year.
So let's clear things up,how the procedure is going on.At this time,the most reachable way for me is Fleaker's idea on reacting it with oxygen:
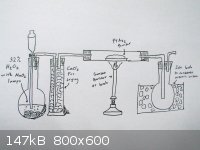
the above setup is what I imagine currently.
Oxygen is generated from 32% hydrogen peroxide with small chunks of MnO2,perhaps my pyrolusite ore.Oxygen ore is channeled to gas washing jar,which
contains anhydrous calcium chloride to dry it.Then it is channeled to long horizontal tube which contains PtAs2 powder in it.
PtAs2 powder will be heated to react it with oxygen.The product (arsenic oxides) will be distilled to another flask immersed in ice bath to condense
it.This receiver flask (and tubes) will be leached with either sodium hydroxide or sodium sulfite solution to get sodium arsenate solution which will
be treated later.
How is this sound?is there any change to make it better here?
|
|
|
unionised
International Hazard
    
Posts: 5126
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Plante,
I don't think a reducing agent like sulphite would help here.
I would use a small pump like the sort used for aquaria to pass air over the stuff rather than using oxygen from H2O2.
I'd also put a second trap after the ice bath. A trap with sodium hydroxide solution would be sure to catch the last of the As.
I'd also not do this unless I was really sure what I was doing.
[Edited on 22-4-11 by unionised]
|
|
|
kuro96inlaila
Hazard to Self
 
Posts: 96
Registered: 21-6-2010
Location: Malaysia
Member Is Offline
Mood: Quietly thinking
|
|
so we go with sodium hydroxide?
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Pure oxygen isn't necessary at all, air oxygen will do, as unionised suggests. That's how roasting is done and that simplifies things at least a bit.
The crucial step is to capture ALL the As2O3, as well as mking sure the remainder of the ore is largely As-free.
Because you’re dealing with a seriously dangerous substance, you can make no easy or lazy assumptions.
For instance, pumping As2O3 vapours through an NaOH solution may sound easy peasy but it’s far from so. Your gas is very hot, remember? It would
make your NaOH washer solution come to the boil immediately, so the air/As2O3 mix needs to be cooled firstly. Run it through an empty washer bottle
immersed in cold water for instance. Much of the As2O3 will condense in it, the rest of this toxin can be captured by the NaOH wash bottle. Use strong
NaOH for maximum capacity and add a second wash bottle, identical to the first, after the first (two wash bottles in series).
Your apparatus needs to be of high quality and seriously air tight and properly secured (stable). Fittings and joints subjected to high temperatures
must be able to withstand those for prolonged times. Cobbling something together from ‘stuff lying around the house' WILL NOT DO.
After roasting, by no means assume your roasted ore is completely arsenic-free. The next step would probably involve digesting the roasted mineral in
aqua regia. Most of the Pt should dissolve in it but arsine may be formed during that step. You need some way to capture all the arsine, 100 %. But
even if no arsine is formed but some As was left in the roasted ore after digestion in AR, you’ll have AsCl3 in solution alongside the PtCl4. After
separating out the Pt (as (NH4)2PtCl6 e.g.) the remainder of the solution and everything that’s been in touch with it is basically potentially
highly toxic waste.
You firstly need also to find as much information on the process as you can muster: none of us here have any experience with roasting/treating an
arsenic bearing mineral and our advice is theory based, not borne out of practical know how.
Start with very small quantities, then ramp up if successful.
The more I think about it, the more I’m convinced this is a job for a very experienced chemist and I still fail to see how you would process 4 kg of
this stuff as an amateur and without being a serious polluter with a deadly by-product
[Edited on 22-4-2011 by blogfast25]
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by blogfast25  | | For instance, pumping As2O3 vapours through an NaOH solution may sound easy peasy but it’s far from so. Your gas is very hot, remember?
|
This is a misunderstanding of the proposed apparatus. The idea is to condense As2O3 vapor to solid, to
desublime it, as it were. The leaching step happens after recovery. This seems like a reasonable idea. It's also clear, however, that a single stage
condenser just isn't going to be efficient enough. Even after you've condensed it all, there are still particle fines to worry about. So it's
reasonable, but it still shouldn't be underestimated. All your other concerns about subsequent stages still apply.
I do have an idea about how this might be tried safely:
Perform the procedure in a completely sealed glass environment. That means glass blowing skills, so that might be off the
table. Even with such a sealed system, there's still the risk of breakage. The idea, though, is just to fix everything in place and fuse it there,
much like working on a vacuum manifold. Annealing is done in place.
Do it under reduced pressure. The reason for this is that a condenser for As2O3 in vapor form acts much like a pump, but only generates enough
pressure difference if other gases are absent. This ΔP enables thinking about a fully sealed system with mass transport provided by
(essentially) a heat engine. The most direct way is to use tank oxygen, but that has all sorts of practical problems about getting the gas metered
into the apparatus.
Use a chemical oxygen source. It must be one that won't give off non-condensing gas, so nothing with nitrogen (N2) or carbon (CO and CO2) or
hydrogen (H2) or chlorine (Cl2) or sulfur (SO2). My only candidate is phosphates, where you reduce the P and oxidize the As. Even if the P reduction
doesn't go all the way to elemental P, you could conceivably still get some oxygen transfer. ΔG doesn't have to be favorable, because you're
driving off gaseous products. The combination of all these three would allow a small-scale experiment to be done with an inverted U-tube, with a
T-branch at the top for vacuum evacuation and seal-off. It still requires glassblowing and a vacuum source, but that seems like a lower barrier to a
safe experiment than other apparatus I can imagine.
|
|
|
Fleaker
International Hazard
    
Posts: 1252
Registered: 19-6-2005
Member Is Offline
Mood: nucleophilic
|
|
I'd really wish he'd just desist. This is very dangerous to do and is NOT for someone with no experience in handling this material. As it stands, he'd
probably get more money for the sperrylite on eBay international.
Sperrylite itself isn't really toxic and can easily be shipped as a mineral specimen.
I wish Chemrox would take a look at this...sperrylite has several different grades; is all of the ore a crystalline mass? It's likely not all PtAs2.
Neither flask nor beaker.
"Kid, you don't even know just what you don't know. "
--The Dark Lord Sauron
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Watson:
Yes, fine particulate matter (As2O3) is likely to be a problem on (de)sublimation. Industrially, electrostatic removal of the dust particles or by
means of cyclones would probably be used. Capturing such a 'mist' in NaOH solution may not even work AT ALL.
|
|
|
Xenomorph
Harmless

Posts: 17
Registered: 23-4-2011
Member Is Offline
Mood: No Mood
|
|
I am mineral colector and I can assure that sperrylite specimens of this quality are worth much more than platinum they contain and also have
significant scientific value. Please, please, please do not destroy them, I beg you! You can not imagine how much it hurts my soul to se such
specimens being destroyed! You can put them on ebay and get enough $ to buy at least 10 times more platinum. Good sperrylite crystals can be sold for
many thousands of $. I hope this will change your mind
http://www.minfind.com/mineral-20513.html
I also would be interested in buying one of them but have not much spare money right now.
Best regards,
Xeno
|
|
|
arsphenamine
Hazard to Others
  
Posts: 236
Registered: 12-8-2010
Location: I smell horses, Maryland, USA
Member Is Offline
Mood: No Mood
|
|
re: meso-dimercapto succinic acid
It is used to chelate arsenic in blood plasma and in the GI tract.
|
|
|
| Pages:
1
2 |