| Pages:
1
2 |
Jenks
Hazard to Others
  
Posts: 163
Registered: 1-12-2019
Member Is Offline
|
|
Building an Analytical System for Plant Alkaloids
I am starting a project in a few weeks that has been on my mind for most of my adult life - there needs to be a robust, open-source system that a
hobbyist or growing professional can put together for themselves to provide analytical services. I know this sounds very general, but the specific
application I have in mind could be applied to an extremely wide variety of common problems.
In this particular case, there are a few factories that have been set up to extract voacangine from the bark of the Voacanga africana tree that has
habitat in many African countries, isolate it and have it converted into ibogaine, which is used primarily in the treatment of addiction to opiates
and stimulants. I have spent much of my life developing these processes and openly publishing the manufacturing procedures. But the process is
hampered by the lack of an easily available analytical tool to assess the amount of voacangine or ibogaine present in intermediate stages of the
manufacturing process.
So in two weeks I intend to go to Ghana and try to cobble together an analytical system using parts I have mostly amassed through eBay, mostly from
the Chinese factories that seem to have down producing electronic and related high-tech parts.
I am following the obvious course of using a low pressure chromatography column of some sort, feeding the effluent from that into a flow cell made of
quartz, passing through it a beam of ~250nm UV from a UV LED, detecting it with one of the specific UV sensors I have bought, and getting the signal
from that into a computer where I will process it in a Linux operating system environment, hopefully taking advantage of existing open-source software
to present the analytical results in a convenient format. Hopefully TSA will allow my suitcases stuffed with these supplies to pass through security
as they have graciously done in the past.
The specifics of this system are where I can use all the help I can get. I have tried to guess the best technologies to put in these bags, but,
although I have a PhD in organic chemistry I'm not even a practicing chemist and need a lot of help. I will try plain silica of course but neutral or
basic alumina seems like a good option for these alkaloids based on the literature and past experience, but there are also functionalized media that I
don't understand very well and the idea of ion exchange chromatography of the cations seems promising. Then there is GC, which has worked with these
alkaloids despite their mass. I don't have time to investigate everything. I have only five weeks in Ghana to get this together, so I need to center
on the best ideas quickly to offer the best working system to everyone when I am done. Thank you for any recommendations you find to share.
|
|
|
Sulaiman
International Hazard
    
Posts: 3754
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
Not my area at all, but may I suggest that someone in Ghana orders equipment and supplies for traditional wet chemical quantitative analysis as backup
/ reference.
Hopefully to arrive about the same time as you.
I guess that you have better info.
(and some members here have real expertise)
https://www.google.com/url?sa=t&source=web&rct=j&...
https://www.orientjchem.org/vol29no2/qualitative-and-quantit...
etc.
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
leau
Hazard to Others
  
Posts: 122
Registered: 3-12-2021
Member Is Offline
|
|
This document might be helpful:
https://www.researchgate.net/publication/257907428_Analysis_...
as chromatography is almost certainly your best choice.
[Edited on 3-7-2024 by leau]
Attachment: Analysis_of_alkaloids_from_different_chemical_grou.pdf (2.5MB)
This file has been downloaded 162 times
|
|
|
Anthracene
Harmless

Posts: 9
Registered: 4-12-2023
Location: Stone Island
Member Is Offline
Mood: Curious
|
|
The system you describe would take care of separation and detection
/quantification at once, pretty neat but also complex.
How about a two step approach?
First separation on a column, maybe there is a suitable SPE column solvent combination that works. You could build this from a syringe, some cotton
and a suitable stationary phase.
Then in a second step UV detection in a cuvette, like measuring OD600 of a culture.
Of course it is more tedious and requires more time to do, but it is a lot simpler to set up.
Even simpler would be a specific reagent to mix directly with the sample and then measuring the absorbance at a specific wavelength. I am thinking of
something like ferrozine for iron(II) or o-toluidine for glucose.
Edit:
Actually, looking at the molecules, they might have some unique absorption bands as they are. Maybe just putting them in a photometer might do the
trick. I did not find any good spectra with 5 min of googling but they might be published. If not, I have two spectrophotometers and could provide
them given a sample. If the goal is an open source device this might be accomplished with LEDs of specific wavelength and photoresistors. Can you
describe what kind of sample you are looking at? Raw plant material will require extraction of course.
[Edited on 3-7-2024 by Anthracene]
|
|
|
bnull
National Hazard
   
Posts: 540
Registered: 15-1-2024
Location: The top of a hotplate again
Member Is Offline
Mood: Molten
|
|
Quote: Originally posted by Anthracene  | | If the goal is an open source device this might be accomplished with LEDs of specific wavelength and photoresistors. |
You can even use LEDs in place of the photoresistors.
|
|
|
Jenks
Hazard to Others
  
Posts: 163
Registered: 1-12-2019
Member Is Offline
|
|
Thanks for the article suggestion - it does look relevant, and it is even accessible! I will check it out.
My work in this area has focused on making technology more accessible in terms of price, otc availability of parts and materials and making the
system easily understandable to set up and use. Past work was on ibogaine extraction or manufacture, but I tested conditions for GC and HPLC for others to reproduce and also to put some numbers behind the production processes.
While I could make a manual process to extract and isolate voacangine and ibogaine from a sample and then quantity it spectroscopically, I imagine a
more elaborate system that would allow convenient, ongoing sample analysis compatible with the needs of the few ibogaine factories in operation today.
These tend to be run by individual chemists or small teams who have dedicated their careers to this work, and I imagine the analytical chemist being a
full time part of such a team and hopefully collaborating with other teams to meet their analytical needs.
I had to pick between gas and liquid chromatography to develop for this project, and I picked liquid mainly out of familiarity, and hopefully I won't
regret it. GC is promising for these alkaloids because I have seen it work, and it may be very selective because many of the alkaloids needing to be
separated are dimeric or of higher molecular weight.
I envision this system delivering solvent at a known, controlled flow rate, either utilizing a pump such as a peristaltic or syringe pump or by measuring the flow rate, perhaps by counting drops optically, and using something like a solenoid valve to control the flow.
The procedure I remember using to prepare samples for HPLC was to weigh 10-20 mg of plant or solid alkaloid mixture directly into each of three 10 mL
volumetric flasks, fill with methanol, sonicate, filter through a syringe filter disk, then dilute to the proper analytical concentration using a
micropipetter and another volumetric flask.
Since this chromatography would operate close to or at atmospheric pressure, the column would be larger than an HPLC column and the injected volume to
be analyzed would be around 0.1-1 mL, which might be measurable using a standard hypodermic syringe, provided that the plastic it is made of is
compatible with the solvent injected. Perhaps it could be injected through a rubber septum into the line leading to the column, or even into a rubber
tube carrying the solvent.
The column could be a small conventional column or a prepackaged one. For the detector I bought a Gilson 111 UV detector, but I plan to compare that with building a flow cell detector using a 1mm quartz flow cell with a 250 nm UV LED, comparing various detectors such as this photodiode or a UV detector module. Since these alkaloids absorb UV longer than 254 nm because of their bicyclic indole system, a 270 nm UV LED and detector can
be added to the same flow cell and may allow some distinction of which alkaloid is passing through. I plan use a Raspberry Pi to process and integrate
the signal from the detector since I am familiar with using them, and that same Pi can be used to manage solvent flow rate and any other automated
features of the system.
The quartz flow cell is expensive, so although I sprung for one I would still like to try my original idea of fabricating one by cutting the bottom
off a 1mm quartz cuvette and attaching tubes to the ends, or even just using quartz tubing itself.
Finally, I am playing with the idea of automatically recycling the solvent by continuous distillation, perhaps using a Peltier module and a pair of CPU cooling blocks to distill eluent from the hot side of the module to the cold side.
[Edited on 4-7-2024 by Jenks]
|
|
|
Anthracene
Harmless

Posts: 9
Registered: 4-12-2023
Location: Stone Island
Member Is Offline
Mood: Curious
|
|
I looked at your homepage and I am really impressed. It is fascinating how dedicated you have been over the years and how much research you have done
for this cause. I see now that you are dealing with quite complex mixtures of alkaloids, so you definitely will need a chromatography step.
For the pumps I think a syringe pump is the best option. Both peristaltic and syringe pumps are theoretically suited but especially the cheap
peristaltic pumps produce fluctuating pressure. You could try to get away really cheap with a big syringe actuated by a spring or rubber bands.
The idea to use UV LEDs and UV index detector modules is great, potentially this could be a ridiculously cheap UV detector. Are you sure that the 2 mm
path length is enough though? I am sure you have some actual data on how well these molecules absorb in the given wavelength. During my lunchbreak
today I found out that quartz discs can be purchased for a small price, maybe a chamber resembling a commercial UV detector module could be
fabricated? Basically a tube with a quartz disc and a rubber seal on either side. This way you could get a path of an inch or more.
The idea of recycling solvents is great, but it seems to me that collecting over a bunch of samples followed by simple destillation is more straight
forward than an automated continuous system.
I wish the best of luck to you and your project in Ghana!
|
|
|
Jenks
Hazard to Others
  
Posts: 163
Registered: 1-12-2019
Member Is Offline
|
|
Thank you, Anthracene, for your feedback and encouragement - it goes a long way. I bought two rather heavy Tecan Carvo syringe pumps. If I can figure
out how to get them working, I agree with you that they should at least be designed to provide a more measured flow rate. The potential problems are
that neither these nor the peristaltic pumps are designed to pump organic solvent - the peristaltic pumps being only as compatible as the probably
silicone tubing they came with - so I am on my own to assess compatibility. And while syringe pumps are the technology that provide continuous,
measured, high-pressure flow for HPLC, those have two syringes pumping in tandem to accomplish that. Since my repurposed pumps have only single
syringes, it is not physically possible for them to provide continuous flow unless I employ a buffering system, but then that system would require the
actual flow per minute to be calibrated.
As for the flow cell path length, these are actually 1mm, not 2mm, which I selected to reduce the size of column needed to have the peaks of interest
be contained within the volume of the flow cell. But in case the path length is inadequate, I bought several standard 1cm quartz cuvettes I can cut
the bottoms off to fabricate 10mm path length flow cells. I also have a sheet of UV filter glass I can cut up to fabricate flow cells the shape I want. I don't know why that sheet is much cheaper per unit area than the quartz
disks you mention, or why any quartz on eBay seems much more expensive than oven door window material, unless I am missing something.
My main constraint seems to be having few choices of chromatography media to test. Even the pre-packaged amine-functionalized medium I linked to above was lost by the seller and refunded, so all I have to test are silica gel and some neutral alumina I can bring. I should have
invested in some C18 cartridge but I didn't see anything at a reasonable price when I had the chance, and the point of my system is to bring the price
of it within reach of us mere mortals.
I have to agree that for an analytical system, maybe it adds unnecessary complexity to recycle solvent automatically. Two more reasons I want to do
this are that it would allow time to leach off any crud on the column that might be detectable so that the column can be used as long as it keeps its
ability to separate components. The other thing is that I would like to be able to use this same technology to automate production chromatography,
using the aforementioned solenoid valves for sample collection. I suppose with only six weeks to work I must be dreaming, but if I can sufficiently
inspire an apprentice, maybe they can fulfill the vision after I am gone.
[Edited on 5-7-2024 by Jenks]
|
|
|
Jenks
Hazard to Others
  
Posts: 163
Registered: 1-12-2019
Member Is Offline
|
|
A little update - I have arrived Safely in Ghana with myself and my copious supplies intact. Another little good news is that the person I am working
with here happens to have basic alumina available, so this will add a third chromatographic medium to the silica gel and neutral alumina I already
can test. I will post again when I have something interesting to report.
|
|
|
Jenks
Hazard to Others
  
Posts: 163
Registered: 1-12-2019
Member Is Offline
|
|
So far I have successfully connected the Raspberry Pi A I brought to my Ubuntu laptop through an ethernet switch not connected to a local or external
network, but internet access was provided by the Pi through the laptop. If anyone would like details on how this was done, please ask.
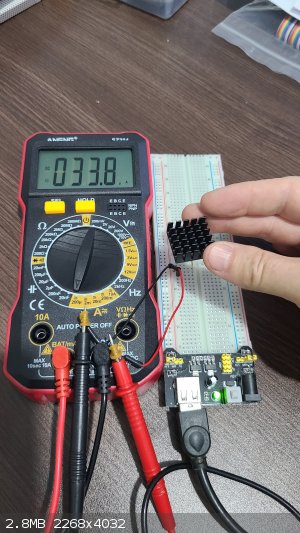
I have also started testing the 250 nm UV LEDs that I bought. One is 0.1 watt with no lens, with an unspecified wide angle, on a 16mm board, rated at 6-7 volts at 0.1 amps. This was attached to a heat sink using thermal glue. I found that it just barely started shining at 5 volts as shown here:

The meter is showing the current produced by an OSI Optoelectronics UV-015 Inversion Layer UV Photodiode, 0.5 microamps in ambient light. When I expose the photodiode to the faint 250 nm UV from
the LED, I get 33.8 microamps:
So my initial plan is to place the LED and photodiode on either side of a quartz flow cell to create a detector for aromatic organic compounds flowing
through in solution.
I also wanted to make a 250 nm UV lamp for my friend Affo to use to visualize TLC. The LED above isn't bright enough even at seven volts. However, a
1W LED with a lens giving a 60 degree beam is sufficient:

The blue voltage converter is raising the 5 volts of the battery bank to 6.2 volts for the LED, and is adjustable using the gold knob of the trimmer. I was
happy with the cost of this setup, as the voltage converter cost $5.65, the LED was $13.69 and the heat sink was $0.35, for about $20 total.
|
|
|
Jenks
Hazard to Others
  
Posts: 163
Registered: 1-12-2019
Member Is Offline
|
|
I got around to testing the cuvettes and the flow cell that I bought for this project. What I purchased from eBay are four 1cm quartz cuvettes from chenting2018, one 1cm quartz cuvette from osag7667, one 1mm quartz cuvette from labsciware and one expensive 1mm quartz flow cell from quartzcrystal. I mention the vendors because of the problem I discovered with the four 1cm "quartz" cuvettes from chenting2018:
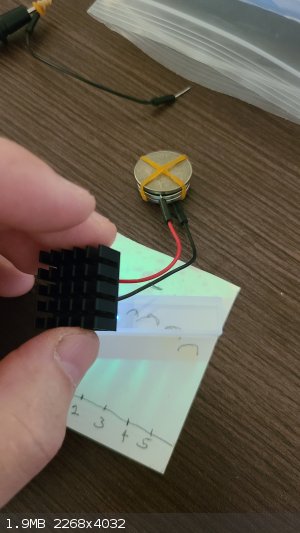
As you can see, the 250nm UV from the LED is not penetrating the cuvette, so that a shadow is cast on the TLC and only the UV going past the cuvette
is causing it to fluoresce. In contrast, the 1cm quartz cuvette from osag7667 transmits UV properly:

Thankfully, the more expensive and useful 1mm cuvette and 1mm flow cell transmit UV like the single 1cm quartz cuvette that I have. My intention with
the cuvettes, to make them into flow cells, it to cut off the bottoms with a Dremel carbide cutting disk and attach glass tubes to the ends using
polyolefin heat shrink tubing, which I hope will seal, be inert, and not swell when exposed to solvent.
|
|
|
Jenks
Hazard to Others
  
Posts: 163
Registered: 1-12-2019
Member Is Offline
|
|
I tried cutting the ends off three of the four glass cuvettes with a carbide disk on a Dremel:

The first one, at the bottom, I started on one corner and the bottom shattered off uncleanly, but at least there are no cracks on the body of the
cuvette. For the middle one I tried working my way repeatedly around the cuvette, with the thinking that when the bottom cracks off that the crack
would follow the scoring line. This seemed to work, but there is a crack coming up from one corner that could eventually cause the cuvette to shatter.
After reading and watching everything I could find online about cutting quartz tubing, I tried dipping the cuvette into water every few seconds as I
worked by way around the cuvette. The cracks seem to follow the cut lines better, but maybe I was lucky, and the job is still ugly because I can't
follow the same line repeatedly while holding the tool and the piece by hand.
Can anyone offer advice on a better way to do this? I have one more glass cuvette to practice on before I turn to the two quartz cuvettes. Scoring and
thermally stressing, I am told, doesn't work well for quartz due to a lower coefficient of expansion. One thing I could try is grinding the bottom off
the cuvette with an abrasive wheel. I don't mind if it takes an hour, but I am concerned that the long grinding would only increase the chance of
cracking, and I couldn't find any online material aside from grinding quartz countertop or faceting quartz stones.
|
|
|
bnull
National Hazard
   
Posts: 540
Registered: 15-1-2024
Location: The top of a hotplate again
Member Is Offline
Mood: Molten
|
|
Try sandpaper. It sounds ridiculous, but here it is: make small disks from sandpaper, attach them to the Dremel, and grind only the regions where the
bottom unites with the walls.
The kind of sandpaper I use is waterproof silicon carbide for metal, usually black or gray. Grains 80, 150, 320 and, if really needed, 1500. But
usually I can get away without this one and it may be a little hard to find. The sandpaper goes on top of a metal disk or that cutting disk you said,
with a rubber disk between them. The rubber (from a bicycle inner tube, for example) is to minimize physical shock.
Use a low speed and water to wet the disks and wash the cuvette every now and then.
Begin by grinding the bottom at the edges at an angle (45° is good enough). Use first the 80, then 150 and finally 320. Change from one grain to
another when you see that the surface is smooth enough and is not being ground anymore. When the bottom falls off, grind the edges until they're all
plane and parallel.
I feel that I'm forgetting something. Edit: Ah, yes. Try at first with those three little boxes you cut out of the cuvettes.
By the way, what is the thickness of the cuvettes?
[Edited on 19-7-2024 by bnull]
|
|
|
Jenks
Hazard to Others
  
Posts: 163
Registered: 1-12-2019
Member Is Offline
|
|
Thanks for the reply, bnull. Both quartz cuvettes have 1mm thick walls but the glass ones are 1.25mm according to the questionable seller, but that
looks right. I have sanding disks I can install on rubber disks backed by the carbide wheel. I don't know what grit these disks are, as the contents
listed on the rotary tool accessory kit doesn't even list the sanding disks. We are having our first power failure now since I arrived, so I will wait until
after to test the sanding.
I hope to use polyolefin heat-shrink tubing to connect each cuvette to tubing to create the flow cell, and that I will find the polyolefin tubing to share some
of the chemical resistance of polypropylene and polyethylene.
Update:
I tried the sanding disks but they weren't very effective. I also tried the side of the cutting disk as the sanding disk but it shattered along with
breaking a few more chips off the end of the cuvette. So I tried the 1/2" sanding band (of unknown course grit) on the rubber mandrel and this worked
amazingly well to grind down the uneven edges of the three glass cuvettes from yesterday, as well as leaving a clean smooth edge without introducing
any cracks. I wasn't able to get the edge completely flat but I didn't really try and figured what I got was good enough.
So I confidently tried grinding down the bottom edges of the last glass cuvette, planning to continue until the bottom of the cuvette became detached
from the sides. At this point I should mention my concern with the invisible cloud of silica I must be generating that I should not inhale. Not having
a mask available, I worked outside with the wind at my back and got into a rhythm of dipping the piece in water, inhaling, then working one edge of
the cuvette back and forth against the band, working my way down from top to bottom, at about a 30 degree angle vs. the bottom, for about half a
minute until time for another breath. Unlike the cutting disk, this felt very gentle, and I used little force between the sander and the cuvette. It
seemed to take about an hour until I figured I was nearly done, when I noticed cracking:

What seems to be happening is that as the glass along the edge of the base becomes very thin, cracks form along the thin edge that can continue into
the bulk of the glass, and have propagated into the cuvette, making this the most unsound of the jobs so far. I think the best method, based on what I
have learned so far, is to work my way around the base of the cuvette with the cutting disk to lead cracks along that path, take the uneven cut I get,
then grind the bottom end as flat as possible with the sander, which will smooth it.
[Edited on 19-7-2024 by Jenks]
|
|
|
bnull
National Hazard
   
Posts: 540
Registered: 15-1-2024
Location: The top of a hotplate again
Member Is Offline
Mood: Molten
|
|
It was an idea. 
|
|
|
Jenks
Hazard to Others
  
Posts: 163
Registered: 1-12-2019
Member Is Offline
|
|
It was a good idea, and I appreciate it. It was useful to even out the end of the cuvette, so I will still use it.

This is an iboga farm I visited today on the outskirts of Accra, Ghana. It wasn't known until a few years ago, as far as I know, that iboga could be
cultivated in Ghana, since it is usually found in Gabon and Cameroon. This farm is relevant to this thread because it is the alkaloids from the bark
of these shrubs, and related Voacanga africana trees, that are to be analyzed with the proposed system.
|
|
|
Jenks
Hazard to Others
  
Posts: 163
Registered: 1-12-2019
Member Is Offline
|
|
I've made a little progress in the last four days.
The Arduino IDE was installed on the Ubuntu 24.04 workstation simply by installing the "arduino" package from the command line:
sudo aptitude install arduino
Right off I couldn't find it among the serial ports. This turned out to be due to a conflict with a braille e-reader, as explained in this StackExchange post, and was removed with the command:
sudo apt remove brltty
At this point, what I have done is to connect two of the analog inputs of the Arduino Uno to two of the UV photodiodes which will measure the UV
passing through the finished quartz flow cell from a 250 nm LED and a 270 nm LED. The power for the LEDs is controlled by two of four relays on a relay module, which itself is controlled by two of the digital outputs on the Arduino. A digital input on the Arduino is connected to a pushbutton
switch which is connected between a 100k and 1M resistor so that if that digital input should fail to be configured as an output, the output won't
draw enough current to burn it out.
The power supply for the UV LEDs was a challenge. I didn't realize that the two kinds of small voltage converters I bought only step voltage down, not
up, so that the five volts from the Arduino can't drive the LEDs. I built the IC 555 Voltage Doubler Circuit with High Current Output described as
project #6 here, but although it converted five volts to 7-8 volts without any load, with the load of the UV LEDs the voltage dropped to five volts. I had to
substitute S8080 and S8550 transistors for the BD139 and BD140 transistors proposed in the design.
Since that didn't work, and the shipping time to order any electronics exceeds the time left for the project, I was glad to be offered a broken TV to
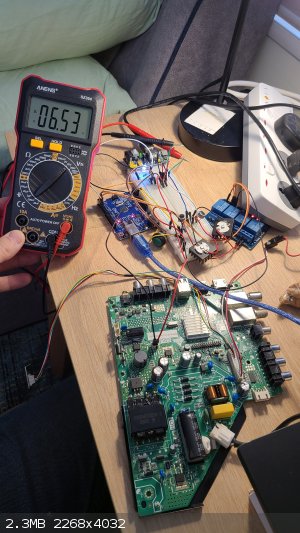
cannibalize for its main circuit board, which apparently includes a regulated 12 volt power supply. That was attached to a power converter I have, which I adjusted to get 6.63 volt output:

When I turn on the LEDs using the aforementioned button, the voltage only drops to 6.53 volts:
A couple nasty things about this TV board are that the big capacitor there charges to 330 volts to run the switching power supply, but retains its
charge when the unit is unplugged. I soldered a 1M resistor across the capacitor so that the voltage drops about 2 volts/second after unplugging. I
also discovered with only a minor shock that the 12 volt plug on this board is apparently not isolated from the mains, and that the ground pin on the
power plug is made of plastic. The voltage step-down module also doesn't isolate input from output. So I have to be careful, and hopefully find a nice
wall-wart to use instead in the final design.
So it looks like this system is ready to put in a box. I know it is minimal, but I have to get this thing working and people trained to use it before
I can make it any nicer.
[Edited on 24-7-2024 by Jenks]
|
|
|
bnull
National Hazard
   
Posts: 540
Registered: 15-1-2024
Location: The top of a hotplate again
Member Is Offline
Mood: Molten
|
|
Try a pair of old PC speakers. Their power supply gives 9 V (or 12 V, depending on the model), and you can even use the enclosures to house your
system: Arduino inside the one with the PSU, and the rest of the system inside the other.
|
|
|
Jenks
Hazard to Others
  
Posts: 163
Registered: 1-12-2019
Member Is Offline
|
|
Thanks for the suggestion. I will look for that. I recently happened to scour a few Goodwill stores near my Sacramento, California area home for
computer speakers and their power supplies to help a few high school students in my wife's physics classes with building Rubens tubes and a wine glass
shattering demo. Their power supplies tended to be 5-9 volts. My constraints here in Ghana are a much different retail selection, and a much more
significant hazard in making long trips by car.
I had hoped to import the completed chromatographic data, read in real time from the arduino by a PHP program and saved as a simple comma-separated
values (CSV) file, into OpenChrom, which seems to be the predominant open-source chromatography software recommended for Linux systems. I am stuck now
in figuring out how to simply find and open the .csv file from OpenChrom, as, despite its name, it seems to expect me to select one of a variety of
proprietary chromatography or mass spectrometer data formats to import from, none of which I can recognize as having a simple liquid chromatography
data format. If anyone has experience with this software and can help me, please post here or contact me through the messaging system. Thanks in
advance. The help I could find online was extremely limited.
[Edited on 25-7-2024 by Jenks]
|
|
|
Sulaiman
International Hazard
    
Posts: 3754
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
1) A usb supply + 'power bank' form a nice 5V dc ups for low power.
2) 12V dc at high power can be had from an automotive battery + charger
3) 19V at fairly high power can be obtained from a laptop charger
4) Many voltages at high power are available from desktop PC power supplies.
In order of safety, 3),1),4),2)
................
PS using solar/hydro/wind power may win some points as a 'green' agenda?
(B.S. but some folk fall for it 
[Edited on 26-7-2024 by Sulaiman]
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
Jenks
Hazard to Others
  
Posts: 163
Registered: 1-12-2019
Member Is Offline
|
|
Thanks for the suggestions, Sulaiman. I will see if any of those can be found without much time invested.
I know progress has been slow but there has been a lot going on. Here is my friend Harrison cutting an aluminum pipe to make shims to space the UV LED
boards properly from the flow cell they will be glued to.

The two UV LED heat sinks will be glued together as shown. Three stacks of three aluminum shims will be glued together to make spacers between the
heat sinks and the flow cell. Shown here is the wider stack already drying, placed where the heat sinks are joined, between the LED modules. The
stacks on the left and the right will be glued together next, then glued at the left and right edges of the LED assembly. The LED assembly will then
be glued to the side of the flow cell facing us, and then the photodiodes will be glued by their edges to the back of the flow cell, centered above
the LED diodes. The silicone cement shown is used for everything. Now hopefully I can remember which of those LEDs is 250 nm and which is 270 nm...

|
|
|
Jenks
Hazard to Others
  
Posts: 163
Registered: 1-12-2019
Member Is Offline
|
|
Things are coming along.
I decided not to go with OpenChrom because, despite the name, I wasn't even able to test the trial version because it requires a license just to
download the documentation, and my credit card was declined while trying to purchase a license. Since my goal is to make this analytical system as
accessible as possible, I don't want to depend on closed source proprietary software that might be orphaned sooner or later.
I also checked out UniChrom, which I was finally able to satisfy the dependencies to install on Ubuntu. This time I was pleased to be able to open the simple .csv
file I had prepared and could see the appropriate chromatogram. This software might work, but I still wasn't completely happy. It is produced by a
company based in Belarus, New Analytical Systems, Ltd, which could complicate support reliability or ethical licensing given the Russian invasion of
Ukraine. Furthermore, their Linux version seems like it has been on the back burner for a long time, with almost all their energy going into their
Windows version. The website says the Linux version is still in beta testing, and the software itself says it is in demonstration mode. The link to
license the Linux version directs to the licensing page for their Windows version. Finally, because UniChrom attempts to apply to several forms of
analysis and to be compatible with as wide a range of instruments as possible, it has a high degree of complexity compared with what is required for
the system I have in mind. This complexity will be an impediment to end users who may lack training in the use of analytical software.
After more pondering, I decided to look to software which is free, open source, easily obtained and in long-term and widespread use. One project uses the R statistical software to provide chromatographic analysis. This is a lovely effort. I only balk because I have not learned to
use R, and I don't want my target audience to have to either.
I found an Excel spreadsheet that presents a very attractive HPLC interface with a chromatogram which might have worked, and it even loads into OpenOffice
Calc, minus the preloaded data. However, I notice that the spreadsheet is hosted on a Chinese website, which made me a little nervous to enable the
macros. More importantly, the spreadsheet is password protected so that I can't easily get in to fix it or tailor it to my own project. It seems
possible the authors might help me, but with this inspiration I decided I would develop my own spreadsheet in OpenOffice Calc to import the .csv data
from my PHP program and present the chromatogram and peak data. So far this has gone smoothly, and I have been able to automatically import data which
is automatically updated in a chromatogram.
On the hardware side, the detector is finally ready, having been painstakingly glued together using silicone cement. I just finished installing the
photodiodes after carefully applying the silicone to the thin rims around the lenses.

|
|
|
Jenks
Hazard to Others
  
Posts: 163
Registered: 1-12-2019
Member Is Offline
|
|
Here is the current system:

A lot of my time has been spent on writing and debugging the software to detect the peaks, written in PHP on the laptop, and I have also been slowed by finding a suitable way to connect the flow cell using
only poly-hydrocarbon tubing. Testing has been by using the shadow from my hand to produce "peaks", but today I finally got around to passing an
actual sample through the flow cell. I finally figured out that the smallest diameter polyethylene tubing I was able to buy, which has an outer diameter of only 1/8", fits tightly, but not too tightly, inside the inlet and outlet connectors of the quartz flow cell,
well enough to form a seal. I also like that this tubing, owing to its thinness, is flexible enough that it is unlikely to snap the quartz connectors
off.
So I simply put the outlet tube into a flask for waste solvent and adapted the other end to a plastic pipette with the top cut off to form a funnel.
The system filled easily by adding solvent (acetone for this test) to the dropper/funnel, keeping the waste flask at the same level to limit the flow
rate. Bubbles purge easily from the system thanks to the 1/16" inside diameter of the tubing, and keeping the flow cell vertical, flowing from bottom
to top, as recommended here.
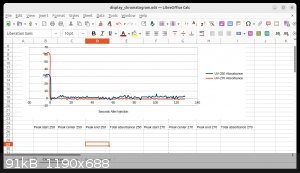
Once the system was full of acetone and free of air, and I could cause the solvent to flow by simply adding more to the "funnel", I ran a test on 1 mL
of 1% toluene, flushed through the system with acetone. I expected a strong peak, but seeing none I ran it on 0.5 mL of straight toluene. Here is the
sorry result:
On the left is where I signal to the software that the sample has been "injected", which causes the Arduino to turn on the UV LEDs. What I expected to
see was a strong peak, at least for the 250 nm detector, that should have blocked out all the UV while it passed. I interpret the complete absence of
a peak as the detectors picking up almost entirely the not-very-bright visible light from the UV LEDs, so that the actual UV from the LEDs, which we
know is there from the TLC fluorescence, was not detected. Given that the UV LEDs cause the TLC to fluoresce more brightly than they are illuminated
by visible light from the LEDs, I didn't think the visible light would be such a problem.
I happened to bring a sheet of shortwave UV selective glass which I can hope to cut to make a filter for the photodiodes, if I can get them safely off
the flow cell. I might use the occasion to connect the photodiodes to amplifiers. Yesterday I learned that the LM1458n dual op-amp ICs that I bought
were not the best choice because they are intended for use with a dual power supply - one with positive and negative voltage and a ground in between.
What I, and most people, have is a single pole power supply which has positive and ground. Using the LM1458n will complicate my circuit a little. I
should have used LM358.
Update: Here is a little test with the filter glass and 1mm quartz cuvette containing 1% toluene in acetone between a 250 nm LED and silica TLC with
254 nm fluorescent indicator. As expected, the UV is blocked by the toluene but passes through everything else, including the cuvette above the level
of the solution. At least my previously untested filter glass is legit.

Update:
That ZW33 UV filter glass doesn't like to break along the score lines! I got some very poorly shaped pieces when trying to cut 12mm off the end of the
piece. I will try grinding them down to small pieces that will fit on the individual photodiodes.
I remembered the responsivity of the OSI UV-15 photodiode being several times higher for visible light, and even IR, than for shortwave UV according
to the datasheet:
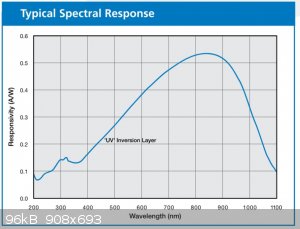
Update 8/4/24:
Things got better this morning, once I tried the Dremel on the UV filter glass. I had planned to use the carbide cutting blade to score the pointed
protrusions to create clean rectangles, but after running past the score line just a couple times, the pieces broke spontaneously along the line. When
I tried trimming the edge of the main piece it took much longer, probably because my unsteady hand made the score line wide, but it was still decent
in the end because the sanding drum accessory smoothed the edges and ground down small protrusions to make them as straight as my hand would allow.
More practice scoring by hand may have led to better results, as demonstrated here. I then used the sanding drum, switching to the smaller diameter drum, to make grooves in the ends of the main piece so it fit snugly against
the face of the flow cell. The silicone I applied to the corners is now curing:

One site I found reported damage to "Hoya" glass, which is what I have according to that site, from the slightest exposure to water. Higher grade Hoya
glass is said to have a layer of quartz to prevent this. My sample must have such a layer, as washing it or soaking it in water led to no visible
change.
[Edited on 4-8-2024 by Jenks]
|
|
|
Jenks
Hazard to Others
  
Posts: 163
Registered: 1-12-2019
Member Is Offline
|
|
I think I've finally finalized the design of the amplifier for each of the photodiodes, using parts I have.
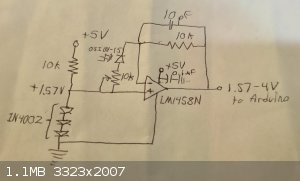
The design is based on a simple, common design depicted as the first stage in the first stage of the amplifier on the photodiode datasheet:
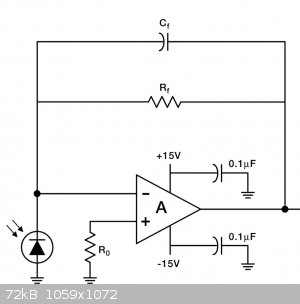
I did not want to build a bipolar power supply, which the LM1458N op-amp is designed for, and the inputs and output of that op-amp are not designed to
go "rail to rail", meaning, in this case, that voltages close to ground or five volts will not be properly amplified, and that the range of the output
will be something less than 0-5 volts. Testing the op-amp as a voltage follower with the variable output provided by a potentiometer, I found the
linear input range to be 1.5 to 4.4 volts. Unfortunately, this means that only 58% of the input range of the arduino will be utilized, with a
corresponding loss of resolution. This is necessary because I didn't pick a more suitable op-amp like the LM358.
To bring the input range above 1.5 volts I used a series of three 1 watt rectifier diodes, which maintain a voltage of 1.57 volts across themselves. A
voltage regulator would have been a better choice, as this voltage will vary with temperature. Fortunately, the climate in Ghana has a very stable
temperature, at least during this part of the year.
The 10k resistor on the left limits the current through the diodes to about 30 microamps. The linear 10k potentiometer limits the gain to being the
output voltage below the 4.4 volt cutoff, which is essential because the baseline of the chromatography, and any small peaks appearing, will depend on
accurately measuring small variations in the full-intensity UV reaching the photodiode. I found experimentally that a setting around 1k brings the top
of the output range to about 4 volts.
The other parts, which are standard, are the 10k resistor on top (usually a higher value) which also determines the gain, the 10 picofarad capacitor
across it to prevent oscillation, and the 0.1 microfarad capacitor across the power connections to the op-amp to filter noise from the power supply.
I hope to build this onto a circuit board attached directly onto the photodiodes in the detector to minimize noise.
|
|
|
Jenks
Hazard to Others
  
Posts: 163
Registered: 1-12-2019
Member Is Offline
|
|
I could use some help if anyone has insight. I built amplifiers for both photodiodes before I realized that the photodiode I drew in my schematic
actually has to be oriented the other way, with the anode to inverting pin 2 of the op amp. This is reversed from the manufacturer's proposed design
and other common designs, but it gives the desirable behavior described above, and the standard orientation just slightly decreases the
output on exposure to UV. I don't know if the response is linear but I figured I would have to calibrate anyway. If anyone has insight why this works,
or any warnings I should be aware of in using this circuit, please inform me. Thanks.
Update:
Here is the final design, which I built and tested:
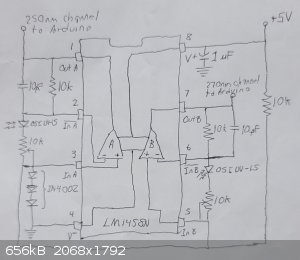
Here is how it looks (I'm ashamed to show the bottom):

Red and black wires are power, brown and yellow wires are first and second potentiometer, yellow and purple are left and right output. I decided to
add wires for the photodiodes so I could test the board before installing it in the detector.
[Edited on 6-8-2024 by Jenks]
|
|
|
| Pages:
1
2 |
|