Niklas
Hazard to Self
 
Posts: 50
Registered: 1-12-2023
Location: Germany
Member Is Offline
Mood: Polymerized
|
|
Synthesis of quinizarin
Greetings everyone, I recently set up my new workbench allowing me to finally do some more interesting chemistry again.
One smaller project I attempted over the last couple of days to get into homechem again was the synthesis of the orange anthraquinone dye quinizarin,
and it seems to have turned out quite well, so I thought I‘d share it with ya‘ll:
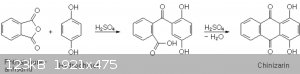
Required chemicals:
-20 g phthalic anhydride (135,0 mmol)
-5 g hydroquinone (45,4 mmol)
-50 ml 96% sulfuric acid (936,1 mmol)
-toluene and glacial acetic acid for the workup
To start of, I added the two solids to a 250ml-RBF, added a stirbar, followed by all of the sulfuric acid. While stirring, 10 ml of dest. water were
then carefully added to the reaction mix, resulting in a orangy-yellow solution containing undissolved anhydride.

A simple reflux apparatus was set up, and I heated the mixture to 180°C for 3 h, followed by 200°C for one additional hour. During this time the
solution turned increasingly more red, by the end it almost looked black, while a lot of the excess phthalic anhydride sublimed forming some pink
crystals in the colder regions of the flask.
   
While still hot the apparatus was quickly disassembled, and all of the reaction mix was very carefully (!) dumped into 400 ml of cold dest. water (+
around 25 ml for rinsing out the flask), I then brought things to a boil to dissolve any excess phthalic acid and other junk, and filtered the brown
suspension though a Büchner funnel while still hot. The brown, like dirt looking residue was kept on the funnel for a few minutes to remove most of
the water, and then the still wet paste was added to another clean flask to dry under vacuum at 120°C afterwards.
 
Well, unfortunately the product, or at least the tar it was mixed in with, really liked to stick to the remaining water due to it being pretty polar,
so after a few hours of heating things and still not being able to fully dry it, I simply dumped in a random amount of toluene into the flask, and set
up a Dean-Stark apparatus to azeotropically remove the water from the product. This seemed to be working quite well, and after around one hour of
refluxing a little less than 10 more ml of water was collected in the receiver.

In the flask a dark red solution of quinizarin remained, with all the polar tar staying undissolved, so while things were still boiling I quickly
disassembled the Dean Stark and, once again, filtered things through a small piece of cotton while hot. Very quickly a mostly orange solid
precipitated, and after cooling things in the freezer over night the crude product could be collected using vacuum filtration, washed with a tiny bit
more toluene, and left on the pump for a few more minutes until all the toluene had evaporated. In total a little more than 7,7 g of quinizarin could
be collected as a reddish-brown solid.
 
The extraction process of the tar could be repeated again to get a little more product, I tried it tho and it really isn’t worth it considering how
cheap the precursors are anyway.
In general the purity of this crude product should already be pretty reasonable, I wanted my quinizarin to be some nice crystals / flakes tho, so to
further purify it I added all of the dry powder to another RBF and topped it of with approximately 96 ml of glacial acetic acid, the exact amount
really doesn’t matter, and again heated things to a reflux resulting in a beautiful dark red solution.
 
After a few minutes of refluxing all the solid should have dissolved, and things were left to slowly cool back down to room temperature, I simply left
the flask in the heat-on so it doesn’t just all crash out as a powder but properly crystallizes. To isolate the product a vacuum filtration though a
Büchner funnel was once again performed, leaving behind a little more than 2,5 g of gorgeous, metallic looking orange flakes, which were left in the
air until no more acetic acid smell could be perceived.
  
Yield: 2,535 g, corresponding to a percent yield of 23,24% (27,5-32% in lit)
I purposely filtered the GAA-solution at a temperature of around 50°C tho, to isolate the beautiful flakes separately, while the rest of the product
precipitated out as something resembling a powder afterwards. So I expect to at least still double my amount of product, gonna keep you updated on
that.
Just as a note, I oriented myself on a Lambdasyn procedure for the most part:
https://www.lambdasyn.org/synfiles/chinizarin.htm
[Edited on 2-4-2024 by Niklas]
|
|
|
Keras
National Hazard
   
Posts: 929
Registered: 20-8-2018
Location: (48, 2)
Member Is Offline
|
|
Nice, nice colour.
I don’t have phthalic anhydride (expensive!), but that’s a nice reaction I'd love to reproduce
|
|
|
UC235
National Hazard
   
Posts: 565
Registered: 28-12-2014
Member Is Offline
Mood: No Mood
|
|
Beautiful synthesis.
|
|
|
palico
Hazard to Self
 
Posts: 63
Registered: 1-10-2013
Member Is Offline
Mood: No Mood
|
|
Hello. Wonderful synthesis and product. The product of first crystallization has the same color of alizarin. Can you make the oxidation of your
quinizarin to quinizarinone ?
|
|
|
Texium
Administrator
       
Posts: 4618
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Very nice synthesis, Niklas!
It shouldn't be. May I direct your attention to my sales thread. I'll sell 500 g of phthalic anhydride for USD $25. Shipping to Europe may be rather expensive, but if you are interested in
multiple items, it could be well worth it.
|
|
|
Niklas
Hazard to Self
 
Posts: 50
Registered: 1-12-2023
Location: Germany
Member Is Offline
Mood: Polymerized
|
|
As Texium noted out, phthalic anhydride really shouldn’t be that expensive. In case you are located in Europe I‘d recommend checking out S3, they
are selling 500 g of it for a little more than 23€:
https://shop.es-drei.de/anhydride/717/phthalsaeureanhydrid-m...
But in anyway, keep me updated on you trying out the synth, would be keen to see your product and yield compare to mine!
|
|
|
Niklas
Hazard to Self
 
Posts: 50
Registered: 1-12-2023
Location: Germany
Member Is Offline
Mood: Polymerized
|
|
Could surely go ahead an try that on a smaller scale. I presume sodium dichromate should work pretty well for that purpose, in case you have some good
literature / procedures on its synthesis I‘d still love to see them tho
|
|
|
Niklas
Hazard to Self
 
Posts: 50
Registered: 1-12-2023
Location: Germany
Member Is Offline
Mood: Polymerized
|
|
Thanks Texium! Was a lot more fun of a synthesis than I initially expected it to be
|
|
|
ItalianChemist
Hazard to Others
  
Posts: 172
Registered: 26-1-2011
Location: Italy
Member Is Offline
Mood: No Mood
|
|
Very nice!
I did this synthesis some time ago but didn't manage to get those beautiful crystals!
|
|
|
ItalianChemist
Hazard to Others
  
Posts: 172
Registered: 26-1-2011
Location: Italy
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Keras  | Nice, nice colour.
I don’t have phthalic anhydride (expensive!), but that’s a nice reaction I'd love to reproduce
|
I have some phthalic anhydride! I could send you some grams for free to do the synthesis if you want!
|
|
|
Niklas
Hazard to Self
 
Posts: 50
Registered: 1-12-2023
Location: Germany
Member Is Offline
Mood: Polymerized
|
|
So, I‘ve been busy for a while now, today I‘ve finally come to filtering the remaining suspension tho.
This ended up with 1,206 g of an orange powder after drying on the pump for a while, adding 13,33% to the percentage yield (36,57% in total, higher
than literature reports).


|
|
|
Dr.Bob
International Hazard
    
Posts: 2750
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
That's a beautiful orange. Nice work and experimental writeup. If anyone in the US wants some Phthalic anhydride, I think I have some left from
making luminol many years ago. Might be partly hydrolyzed, but easy to fix that with some heat and vacuum. Likely also still have some
3-nitrophthalic anhydride also.
|
|
|
Niklas
Hazard to Self
 
Posts: 50
Registered: 1-12-2023
Location: Germany
Member Is Offline
Mood: Polymerized
|
|
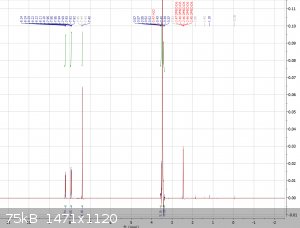
Greetings everybody, today I‘m back with some NMR results of the product. As we can see, the spectrum is just as we would expect it to look like in
the area of aryl peaks, since the cutoff was at 13 ppm we won’t be able to see the two phenol peaks (expected to be at a little more than 16 ppm)
tho. Both the DMSO and H2O peak can be ignored, the H2O peak is unusually high in this case due to me using some old 20 year old d6-DMSO to finally
finish up the bottle, causing it to already have absorbed moisture from the air. Other than the the spectrum seems pretty clean, we can’t even see
any (notable) methyl peaks that may come from small amounts of residual GAA so that’s pretty nice.
|
|
|