Monoamine
Hazard to Others
  
Posts: 168
Registered: 25-5-2021
Location: Sweden(ish)
Member Is Offline
Mood: +7
|
|
Solvents for Lithium Hydride
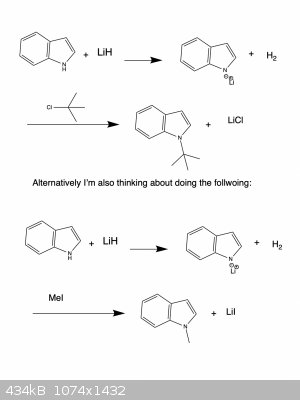
I'm trying to deprotonate the indole nitrogen and subsequently alkylate the nitrogen. I have some LiH which I think should work for deprotonating
indole.
However, I'm having a lot of trouble finding a solvent that will properly dissolve the Lithium hydride and the indole.
The only thing I cam across so far in the literature is DMF. But due to its high boiling point I would rather avoid it...
Do you know of any other solvents (with might work for this but which can more easily be removed aster the reaction is complete?
|
|
|
DraconicAcid
International Hazard
    
Posts: 4356
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
I suspect your first reaction isn't going to work, as you're trying to do Sn2 on a tertiary alkyl halide. You're much more likely to get elimination.
For the second one, does the LiH need to dissolve very much? It might work just as a suspension in, say, THF.
(Disclaimer- not really an organic chemist.)
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2800
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
There are no soluble saline hydrides in any solvents. LiH is always used heterogeneous. And yeah I don't think you can attach a tetr-buryl like that.
|
|
|
bnull
Hazard to Others
  
Posts: 478
Registered: 15-1-2024
Location: South of the border, wherever the border is.
Member Is Offline
Mood: "Ah, what the hell; it's Christmas!" - Carmine Lorenzo
|
|
Quote: Originally posted by Monoamine  | Do you know of any other solvents (with might work for this but which can more easily be removed aster the reaction is complete?
|
The only two solvents that I can remember are DMF, which you want to avoid, and DMSO, which has an even higher boiling point. Other solvents may
promote the alkylation of C3 rather than N.
My suggestion is use DMF or DMSO and then vacuum distill it afterwards. Any indole you synthesize will boil some 70°C above the solvent anyway.
There's this paper: Chemoselective N-Acylation of Indoles and Oxazolidinones with Carbonylazoles. I know it is about acylation but maybe you can adapt
something from it or from its references.
By the way, why are you interested in alkylating the indole? Just for fun or is it college stuff?
[Edited on 1-3-2024 by bnull]
Attachment: Alkylation of indoles.pdf (626kB)
This file has been downloaded 136 times
Quod scripsi, scripsi.
B. N. Ull
P.S.: Did you know that we have a Library?
|
|
|