Synthesis of Orange II
Hello people !
Today we resurrect an old classic of dye chemistry: the preparation of Orange II. It is the first step to a series of experiments leading to 1,2-naphthoquinone.
The reaction involved is the diazotisation of 2-naphthol with sulfanilic acid.
Let's find out !
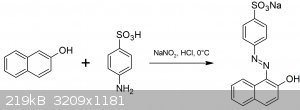
Procedure
A solution A is prepared by dissolving 5 g of sodium nitrite in 10 ml of water.
Solution B is done by dissolving 12 g of sulfanilic acid in 150 ml 0.4 M NaOH aqueous solution, heated to 70 Celsius for a while, then let cool to
room temperature. Solution A and B are cooled to 0 Celsius in ice-bath, mixed carefully. Then cold concentrated HCl is added dropwise to reaction
mixture until pH = 3. Reactio mixture turn orange, then a yellow suspension forms.
Apart, solution C is made of 8.3 g of 2-naphthol dissolved in 50 ml 1.3 M NaOH aqueous solution, heated to 70 Celsius on hotplate for a while, under
stirring. Then it is left get to room temperature, once cool, placed in ice-bath.
Once 0 Celsius, solution C is poured into reaction mixture ( sol. A+B ) under stirring. A deep orange color appears, and quickly a suspension forms.
10 g of NaCl are added at the end, reaction mixture becomes very sticky, not possible to stir anymore. The flask is left in the bath under stirring
until it gets back to room temperature, then gravity filtered. The orange residue, is pressed on paper, let dry in open air overnight. Next day is
pressed again, it is still humid but it cannot be dry in oven because of decomposition.
Yield is 19 g but it is irrelevant. I consider it 100% yield and use directly in the next step.

Discussion
This time I have not so much to discuss. No product characterization has been realized because useless. The Orange II will be directly reduced to
aminonaphthol in the next step.
As usual I link you to the YT video for a more detailed procedure.
References
Org. Syn. vol. 17, pag. 9, 1937
See you nex time, thanks for attention,
palico
|