palico
Hazard to Self
 
Posts: 63
Registered: 1-10-2013
Member Is Offline
Mood: No Mood
|
|
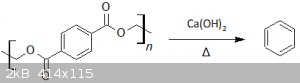
Synthesis of benzene from PET water bottles
Hello people,
today I present a very useful preparation, the synthesis of benzene from PET water bottles. The reaction employed is the pyrolysis of PET pieces with calcium hydroxide. The raw product is then purified by
distillation. According to the reference [1], it proceeds through the initial PET hydrolysis to calcium terephthalate which then decarboxylate to benzene. Toluene, ethylbenzene, xylene
and others are found as side-products.
Procedure
The synthesis is realized in a 11.4 l steel alcohol distiller re-adapted as reactor, purchased from eBay. PET bottles are cleaned and eventually
dried, removed of label, cap, seal, also top and bottom part because too much hard, and cutted in pieces. Then 0.5 kg of PET pieces is mixed with 1.5
kg of calcium hydroxide ( PET:Ca(OH)2 1:3 ), introduced into reactor, pyrolysis started on a gas burner flame. The setup is in Figure.
 
The flame is open to maximum, the reactor bottom is red hot. Temperature of pyrolisate overcome 200 Celsius. Some fumes are collected initially, but
then a yellow-green liquid pass through condenser along with water and wax. After a total of 2 hours, heating is interrupted, and everything let cool
down to room temperature. Pyrolisate is separated from aqueous layer, extracted with 3x50 ml of water. Organic phase filtered through cotton plug to
remove as much wax as possible. A yellow-green clear liquid is obtained which quickly turn brown; crude pyrolisate is 45 g ( 47 ml ), corresponding to
9% of the initial PET mass.
After collecting five batches, 250 ml of that is distilled simply in oil bath at 100 Celsius, discarding the fraction passing below 75 Celsius and
collecting the one between 78-80 Celsius; 95 ml of very clear limpid benzene is obtained, d20 = 0.870 g/ml.

Discussion
Almost 100 ml of very pure benzene are collected from 2.5 kg of PET bottles. Reference report that from 1:5 PET:Ca(OH)2 ratio on, almost pure benzene
is obtained, so theoretically the yield could increase much more.
Anyway, this experiment demonstrate that very valuable chemicals can be got from a trash material as water bottles. The reaction should not be seen
only as a benzene synthesis but also the chemical recycling of PET bottles, which otherwise have to be done mechanically. The higher fractions can be
recoverd as well, adding value to the experiments.
I think this reaction can be scaled up to become economically sustainable. Gas can be recovered and used to fuel the reaction itself, which furtherly
decrease cost.
If biomethane is used as fuel, that add renewability to the process.
Reference
1. Chem. Lett. vol. 33, pp. 282-283, 2004
As usual, I thank you for the attention and remind you to watch the detailed procedure on my YT channel. Here you can find synthesis, purification, flammability test.
palico
|
|
|
dettoo456
Hazard to Others
  
Posts: 255
Registered: 12-9-2021
Member Is Offline
|
|
Nice job. The yield is kind of shit considering the weight of PET required and natural gas but it’s definitely worth it if benzene is harder to get
near you. You can also pyrolize HDPE & LDPE w/ alumina to yield long chain alkanes with potential use in gas engines.
A smaller reaction vessel made out of steel and shoved into a kiln (or just a better insulated heat source) might be more economical and achieve
higher yields as compared to the stove.
|
|
|
Fery
International Hazard
    
Posts: 1052
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
Well done and quite big scale experiment!
Ca salts of organic acids are used for producing ketones. I wonder whether using Na terephthalate couldn't yield more benzene or whether you also did
not obtain substantial amounts of benzophenone.
|
|
|
palico
Hazard to Self
 
Posts: 63
Registered: 1-10-2013
Member Is Offline
Mood: No Mood
|
|
@dettoo456: the yield can be increased with higher PET:Ca(OH)2 ratio according to reference. I could not use 1:10 because the volume of reactor but
1:5 already gave much pure pyrolisate, almost wax free. Is wax which lower the yield. Filtration takes long time, during the which pyrolisate
evaporate quite a lot.
@Fery: other compounds are in distillation residure. But I did not separated.
|
|
|
Hexabromobenzene
Hazard to Others
  
Posts: 151
Registered: 27-4-2021
Member Is Offline
|
|
Have you had problems with clogged pipes? When pyrolyzing PET bottles WITHOUT calcium oxide, a large amount of sublimate is formed from benzoic and
terephthalic acid (mainly terephthalic). This leads to clogging of pipes and explosion of the pyrolysis apparatus in extreme cases
But if you assemble a pyrolysis apparatus that will not clog, then you can make large quantities of benzoic and terephthalic acid for free. Benzoic
acid is separated from terephthalic acid by hot water extraction
A lot of acetaldehyde is formed from by-products. Terephthalic acid can also be contaminated with biphenylcarboxylic acid and alkylbenzoic acids
I feel very sorry for this distillation apparatus. Once the plastic is pyrolyzed, it will become contaminated. Better to use a paint can
[Edited on 26-1-2024 by Hexabromobenzene]
|
|
|
Hexabromobenzene
Hazard to Others
  
Posts: 151
Registered: 27-4-2021
Member Is Offline
|
|
Quote: Originally posted by dettoo456  | Nice job. The yield is kind of shit considering the weight of PET required and natural gas but it’s definitely worth it if benzene is harder to get
near you. You can also pyrolize HDPE & LDPE w/ alumina to yield long chain alkanes with potential use in gas engines.
A smaller reaction vessel made out of steel and shoved into a kiln (or just a better insulated heat source) might be more economical and achieve
higher yields as compared to the stove. |
You can use firewood and wood wastes
|
|
|
palico
Hazard to Self
 
Posts: 63
Registered: 1-10-2013
Member Is Offline
Mood: No Mood
|
|
@hexabromobenzene: yes, acetaldehyde odor is detected at the beginning of pyrolysis. No, I have never pyrolyzed PET without catalyst, and I will not.
This apparatus is dedicated to PET pyrolysis only, do not feel sorry.
|
|
|
ItalianChemist
Hazard to Others
  
Posts: 180
Registered: 26-1-2011
Location: Italy
Member Is Offline
Mood: No Mood
|
|
Very nice setup! Well done!
The final product looks very pure!
|
|
|
dettoo456
Hazard to Others
  
Posts: 255
Registered: 12-9-2021
Member Is Offline
|
|
Moved from other thread:
This paper (https://doi.org/10.1016/j.polymdegradstab.2022.109900) which describes PET pyrolysis to yield Paraldehyde, EG, and Benzoic Acid under fairly
‘weak’ conditions. Those conditions being atmospheric pressure, no catalyst or pH modifiers, and fairly fast (although they say slow) heating to
only 400C. The whole process would only take 13 minutes apparently; 40C min−1 to 200C followed by 25C min−1 to 400C. They don’t state a dwell
time at the 400C as far as I could tell, but in any case it wouldn’t be too difficult to replicate in theory.
A yield of around 24g Paraldehyde, 10.5g Ethylene Glycol, & 4.6g Benzoic Acid are recovered as “oil” from 100g of PET.
I’m looking to maybe try this procedure out in an Al paint pressure canister modified as a retort. Does anyone have any experience with a prep
similar to this (that being PET pyrolysis at lower temps without any catalyst)?
|
|
|
palico
Hazard to Self
 
Posts: 63
Registered: 1-10-2013
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by dettoo456  | Moved from other thread:
This paper (https://doi.org/10.1016/j.polymdegradstab.2022.109900) which describes PET pyrolysis to yield Paraldehyde, EG, and Benzoic Acid under fairly
‘weak’ conditions. Those conditions being atmospheric pressure, no catalyst or pH modifiers, and fairly fast (although they say slow) heating to
only 400C. The whole process would only take 13 minutes apparently; 40C min−1 to 200C followed by 25C min−1 to 400C. They don’t state a dwell
time at the 400C as far as I could tell, but in any case it wouldn’t be too difficult to replicate in theory.
A yield of around 24g Paraldehyde, 10.5g Ethylene Glycol, & 4.6g Benzoic Acid are recovered as “oil” from 100g of PET.
I’m looking to maybe try this procedure out in an Al paint pressure canister modified as a retort. Does anyone have any experience with a prep
similar to this (that being PET pyrolysis at lower temps without any catalyst)? |
That article show another way to get resources from PET. Do you see ? Waste does not exist, but are just resources ?
|
|
|
Hexabromobenzene
Hazard to Others
  
Posts: 151
Registered: 27-4-2021
Member Is Offline
|
|
I do not understand why the authors report about ethylene glycol. Pet plastic during pyrlysis will give you acetaldehyde, benzoic acid and terephtalic
acid. However, you will have a lot of problems due to terephtaliс acid. It forms bung in pipes
See here for more info
https://www.researchgate.net/publication/334529290_Slow_Pyro...
For higher yield benzoic acid you need catalyst
|
|
|
LookingForAnswers
Harmless

Posts: 19
Registered: 25-3-2024
Location: United Kingdom
Member Is Offline
Mood: No Mood
|
|
what do you think about vacuum pyrolysis?could vacuum help degrade polimer to its monomers in lower temp? Im not advanced chemist however, logic tells
me it may work.
Chemistry Pope
|
|
|
palico
Hazard to Self
 
Posts: 63
Registered: 1-10-2013
Member Is Offline
Mood: No Mood
|
|
I have no idea about application of vacuumin pyrolysis, it helps instead nitrogen flushing before reaction.
|
|
|
Mateo_swe
National Hazard
   
Posts: 556
Registered: 24-8-2019
Location: Within EU
Member Is Offline
|
|
Are this just for an intresting method of doing something with old PET bottles?
There is an easy way of making benzene from sodium benzoate (food store) and NaOH (lye).
Nilered has a video of the procedure.
You just mix the benzoate and NaOH in an empty paint can, make a hole in the top and attach a condenser distillation setup.
Then put a caming burner under the paint can and destill crude benzene.
Do this outside.
The crude benzene can then be re-distilled to obtain clear benzene.
I tried this and it works very good.
|
|
|
palico
Hazard to Self
 
Posts: 63
Registered: 1-10-2013
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Mateo_swe  | Are this just for an intresting method of doing something with old PET bottles?
There is an easy way of making benzene from sodium benzoate (food store) and NaOH (lye).
Nilered has a video of the procedure.
You just mix the benzoate and NaOH in an empty paint can, make a hole in the top and attach a condenser distillation setup.
Then put a caming burner under the paint can and destill crude benzene.
Do this outside.
The crude benzene can then be re-distilled to obtain clear benzene.
I tried this and it works very good. |
This method is much easier than sodium benzoate pyrolysis. You need two reactions, while here it is just one.
|
|
|
foxofax474
Harmless

Posts: 12
Registered: 17-4-2023
Location: USA
Member Is Offline
Mood: Still processing
|
|
Quote: Originally posted by palico  | | This method is much easier than sodium benzoate pyrolysis. You need two reactions, while here it is just one. |
Well i mean to be fair, the yields here are suboptimal to say the least, so unless you have a very large amount of PET, the sodium benzoate method
seems much more viable.
(∩^o^)⊃━☆ the proof is by magic
|
|
|
Hexabromobenzene
Hazard to Others
  
Posts: 151
Registered: 27-4-2021
Member Is Offline
|
|
It was found that temperature increases rate alkaline hydrolysis of terephthalates. So at room temperature you have to wait several months for
complete hydrolysis, but at a temperature of 90-100 degrees in the oven the process will take only 5 days.
In this case, you can use excess plastic. All alkali is used.
|
|
|
MrDoctor
Hazard to Self
 
Posts: 60
Registered: 5-7-2022
Member Is Offline
|
|
Quote: Originally posted by Hexabromobenzene  | Have you had problems with clogged pipes? When pyrolyzing PET bottles WITHOUT calcium oxide, a large amount of sublimate is formed from benzoic and
terephthalic acid (mainly terephthalic). This leads to clogging of pipes and explosion of the pyrolysis apparatus in extreme cases
But if you assemble a pyrolysis apparatus that will not clog, then you can make large quantities of benzoic and terephthalic acid for free. Benzoic
acid is separated from terephthalic acid by hot water extraction
A lot of acetaldehyde is formed from by-products. Terephthalic acid can also be contaminated with biphenylcarboxylic acid and alkylbenzoic acids
I feel very sorry for this distillation apparatus. Once the plastic is pyrolyzed, it will become contaminated. Better to use a paint can
[Edited on 26-1-2024 by Hexabromobenzene] |
i believe there is a paper describing a solution to that problem which is to react in a pipe/tube, and then in a segment immediately above it, with a
much higher temperature direct all vapor products through this, resulting in a more complete hydrolysis. i believe silica and calcium hydroxide are
used for that, namely it finishes off any terephthalate that forms. also, apparently terephthalates would degrade among the pyrolysis products so,
ensuring theres no reflux of any sort helps quite a bit too. Im not sure what the specific temperature was but i believe that the calcium catalyst
regenerates by reacting and breaking down the TPA, even though its heated well below the breakdown temp of carbonate.
|
|
|