Fery
International Hazard
    
Posts: 1015
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
pseudoionone by Aldol condensation of citral with acetone
Various methods could be used, different ratio of reactants (ratio of excess of acetone), catalysts (Na methoxide, solid NaOH, 40% sol NaOH in water,
heterogenous catalysts like CaO:MgO), temperatures, methods of purification (vacuum distillation vs. bisulphite adducts), ... Sources for studying
attached at the end.
At first it is necessary to purify citral which is an aldehyde and may undergo oxidation into acid with higher b.p. It is necessary to determine b.p.
of the citral at the level of vacuum of the pump as the same vacuum level will be used further. The vacuum pump used was cheap ebay chinese 2-stage
oil rotary vane vacuum pump.
Isomeric (trans + cis) citral from es-drei.de (Citral Isomerengemisch min. 96%, Food Grade https://shop.es-drei.de/aldehyde/11451/citral-isomerengemisc... ) was vacuum distilled, fraction in b.p. range 60-65 C was collected. Vacuum
pressure unknown (no such sensitive manometer available). 230 g of clear colorless distillate obtained and 13 g yellow remainder in the flask. It was
necessary to remove especially acids from the aldehyde due to its air oxidation. 8% molar ratio of NaOH is going to be used as a catalyst (if 4%
content of acids, then half of the catalyst would be inactivated and the reaction 2-times slower, more reactant unreacted = lower yield, harder
separation). B.p. of the acid from citral unknown, but could be estimated approx 70 C above the citral b.p. which is different enough (e.g. octanal
171 C, octanoic acid 240 C).
The b.p. at which the citral boils under the vacuum level of the used vacuum pump should be known to later separate unreacted citral from the product.
If citral unavailable it could be vacuum distilled (or maybe steam distilled too) from essential oils of lemon myrtle, litsea cubeba, lemongras - list
available here
https://en.wikipedia.org/wiki/Citral
http://www.thegoodscentscompany.com/data/rw1003432.html
citral
3,7-dimethyl-2,6-octadienal
Boiling Point: 228.00 to 229.00 °C. @ 760.00 mm Hg
Boiling Point: 103.00 to 104.00 °C. @ 12.00 mm Hg
http://www.thegoodscentscompany.com/data/rw1005841.html
pseudoionone
3,5,9-undecatrien-2-one, 6,10-dimethyl-, (3E,5E)-
Boiling Point: 114.00 to 116.00 °C. @ 2.00 mm Hg
Boiling Point: 143.00 to 145.00 °C. @ 12.00 mm Hg
boiling points of various compounds and sideproducts at atm. pressure:
diacetonealcohol b.p. 166 C but easy to wash out with water
mesityl oxide b.p. 130 C
isophorone b.p. 215 C, poorly soluble in water (1,2 g / 100 ml) could not be washed out with water
citral b.p. 225 C
pseudoionone b.p. 265 C
Calculations of reactants:
114,2 g citral = 0,75 mol (M=152,24 g/mol)
165 g acetone (molar ratio 3,8:1 acetone:citral) = 2,84 mol (M=58,08 g/mol) density 784 g/l = 210,5 ml, analytical grade, 99,6 % purity, water <
0,30 % https://sklep-chemland.pl/en/odczynniki-chemiczne/odczynniki...
2,53 g NaOH catalyst (molar ratio 1:12 NaOH:citral) = 0,063 mol (M=40,00 g/mol) dissolved in 3,8 g water, (solutio 40% NaOH + 60% H2O)
experiment
In 1 L RBF was thoroughly mixed 165 g of acetone with 6,33 g 40% NaOH and then immediately 114,2 g of citral was added at once under magnetic stirring
at room temperature. Necks of flask were closed with pieces of platic sheets with rubber bands to minimize air oxidation. Then heating turned on and
set to 40 C at the heating mantle. The reaction exothermically overheated further to 50 C at which it was necessary to cool it down to 40 C and then
it was kept at 40 C. The reaction time was 90 minutes measured since the reaction reached 40 C for the first time. Perhaps it would be better to set
heating mantle to only 30 C at the beginning and not to final 40 C, then the reaction would maybe reach the 40 C, but not more, so maybe the cooling
wouldn't be necessary.
Neutralization solution of citric acid was prepared during the reaction. 0,021 mol citric acid monohydrate should be theoretically required for the
neutralization of the whole NaOH. Molar mass citric acid monohydrate = 210 g/mol which is 4,4 g. An excess 7 g of the citric acid was used (because
when using the minimal amount then trisodium citrate would be still weak alkaline). The acid was dissolved in cca 10-15 ml of water (the dissolution
was endothermic but this small amount later easily warmed to room temperature at which it fully dissolved).
After 90 minutes the solution of citric acid was added at once under magnetic stirring to stop the reaction. The neutralization did not significantly
warm the the reaction, it remained at 40 C after that. The color changed from dark red to orange.
Acetone was distilled out from the 1 L flask under magnetic stirring (without stirring there could be immiscible water with organic product at the end
which could bump, stirring should avoid bumping). Heating mantle was set only upto circa 100 C (the T was not exactly measured). Most of acetone was
distilled out at such temperature (some still stayed inside).
Cca 150 ml of distillate collected, no bumping observed thanks to mg. stirring.
When stopped stirring, the content in the distillation flask separated into upper organic layer and small amount of lower water phase (cca 10 ml).
The product was washed 4x with 100 ml of water in 250 ml separatory funnel. It won't be good idea to add water if there was a lot of acetone as then
no water layer separation - too much of acetone would decrease density of water phase thus make its density closer to the density of organic phase,
make both phases more miscible etc... that's why most of the acetone was distilled out previously.
After the last 4th washing the organic layer was let to stay in the separatory funnel overnight, a little more water separated as bottom phase.
Yield 135 g = 0,70 mol (M=192,2 g/mol), very weak lemon scent observed, it could be the scent of unreacted citral or the scent of the product (I do
not know if some of them could beat the other due to scent strength, lower sensitivity threshold, ...).
It was dried with anhydrous CaCl2. Yield 131,8 g.
It was predistilled from 500 ml RBF using water aspirator pump vacuum level (circulating cold winter water), and lazy setup without condenser (only
RBF as a receiver cooled with snow). Cca 10 g of forerun collected. T in distillation flask approx 120 C measured from outside using infrared
thermometer (it could be +-30 C as well). That removed all the moisture present (still few drops colorless of water at the bottom of pale yellowish
forerun distillate), remainders of acetone and some of the condensation products of acetone (mesityl oxide, diacetone alcohol).
To the 500 ml RBF was reattached normal vacuum distillation apparatus with Anchutz-Thiele adapter and the product was distilled using the same vacuum
pump which was used for distillation of the citral.
Fractions collected, all of them pale yellowish unlike colorless citral:
95-105 C circa 25 ml
105-115 C circa 50 ml
115-135 C circa 15 ml
A dark remainder stayed in the distillation flask circa 25-50 ml.
materials to study more:
https://img.perfumerflavorist.com/files/base/allured/all/doc...
https://bpb-us-e1.wpmucdn.com/sites.ucsc.edu/dist/9/291/file...
https://sci-hub.ee/10.1021/ja01666a017
https://www.youtube.com/watch?v=i5-EXDoV1vM
|
|
|
Fery
International Hazard
    
Posts: 1015
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
wow the thread id is 160000
OK photos.
Distillation of citral
  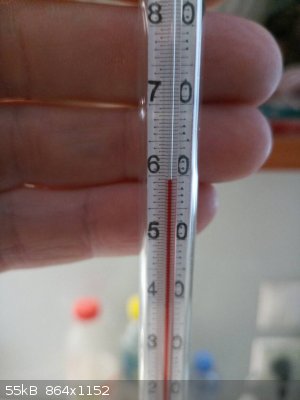  
|
|
|
Fery
International Hazard
    
Posts: 1015
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
apparatus and the start of the reaction

the start of the reaction visible very soon - not crystal clear anymore

reaction progressed further

near the end of the reaction

reaction stopped after neutralization of the NaOH with citric acid and changed color

acetone distilled out and the remainder in the distillation flask, little of water bottom phase visible
 
washing with water in separatory funnel

lazy predistillation using water aspirator pump vacuum level for degassing, removing water, acetone, low boiling sideproducts..., few drops of water
visible in the condensate
 
regular vacuum distillation using the same vacuum pump used for citral distillation
             
|
|
|
Bedlasky
International Hazard
    
Posts: 1239
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Online
Mood: Volatile
|
|
Again, nice work Fery!
|
|
|
|