RU_KLO
Hazard to Others
  
Posts: 248
Registered: 12-10-2022
Location: Argentina
Member Is Offline
|
|
Do i have Potassium Chromate?
Hi,
been trying to make Potassium Chromate. I need more or less pure for qualitative analysis.
I follow wikis procedure for Potassium dichromate (SS + HCl) -> green/blue solution + Sodium carbonate -> mixed iron/chromium hidroxides +
oxidizer > filtrate: yellow solution > removing water to cristallize.
obtained yellow cristalls. (supposedly Potassium Chromate)
regarding oxidizer:
tried H2O2 (3% and 30%) then boiling to remove excess H2O2.
tried clorox, test tube scale, but abandoned becasue of chlorine (do not own fume hood)
also tried potassium persulfate, but this did not give yellow solution filtrate, but clear solution. (and upon adding 6M NaOH and heating a black
precipitare was formed. Sulfide??)
Ok, this is what puzzles me:
made a solution of this salt : yellow solution and upon adding H2SO4 did not change color to red/orange. (this is a qualitative test for chromates
which fails)
tried 96% sulfuric, 30% sulfuric and diluted Hcl (0.1Molar). None of them gave me the red/orange/peach color.
Tried BaCl2 test, by adding some drops. a white/mild yellow precipate was seen. (BaCl2 is not reagent type, it was done from Baryte + Carbonate + HCl
procedure)
it was noted that when adding acid to "the salt" /or solution of "the salt", some effervesence takes place. (like a carbonate. did not analize the
gas)
Does this is normal for potassium Chromate?
Tried 6M NaOH as qualitative test, without good (confirmation) results.
(procedure from here:
group: Al3+, Cr3+, Zn2+, Sn2+, Sn(IV), As(III), As(V)
The group reagent of this group is 6 M solution of NaOH in the presence of 3 % solution of H2O2. Under the action of the group reagent at first the
precipitates of hydroxides are formed, they are dissolved in the NaOH excess. The presence of H2O2 stipulates formation of hydroxo anions and oxo
anions of these elements at the highest rates of oxidation)
"Analytical chemistry (Qualitative analysis) Part I The manual for students of
higher schools O. A. Ievtifieieva, V. V. Bolotov, T. A. Kostina, O. M.
Svechnikova, T. I. Yuschenko, N..." july 2014"
Got some green/blue precipitate, but it readly becomes rust brown upon adding H2O2 and heating)
Probably because Cr6+ instead of Cr3+???
Any help on this regard or if some one has more tests or confirmed procedure, will be thankfull for sharing.
Thanks
Go SAFE, because stupidity and bad Luck exist.
|
|
|
Kloberth
Harmless

Posts: 38
Registered: 2-6-2023
Member Is Offline
|
|
watch explosions and fires video on this topic! he used calcium hypochlorite as the oxidiser.
https://www.youtube.com/watch?v=F_W-IyUTM5M&ab_channel=E...
https://www.youtube.com/watch?v=3uzTNjuUyyk&ab_channel=E...
|
|
|
RU_KLO
Hazard to Others
  
Posts: 248
Registered: 12-10-2022
Location: Argentina
Member Is Offline
|
|
thanks,
but can someone that has potassium chromate confirm:
"when adding acid to "the salt" /or solution of "the salt", some effervesence takes place" by salt mean Potassium Chromate.
thanks
Go SAFE, because stupidity and bad Luck exist.
|
|
|
woelen
Super Administrator
        
Posts: 8083
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
If you add acid to pure potassium chromate, then no gas is produced, nothing at all.
Potassium chromate is yellow. On addition of acid, it turns orange, due to formation of the bright orange dichromate:
2 CrO4(2-) + 2H(+) ---> Cr2O7(2-) + H2O
This is somewhat simplified, in reality there is an equilibrium reaction, which also involves HCrO4(-) ions, but what I have given above is the main
reaction, certainly when pH is low.
|
|
|
RU_KLO
Hazard to Others
  
Posts: 248
Registered: 12-10-2022
Location: Argentina
Member Is Offline
|
|
from Wiki:
Potassium dichromate
"The resulting solution should be dark green due to the chromium(III) ion.Sodium carbonate (not sodium hydroxide) is added to the
solution to neutralize all remaining acids and precipitate a mixture of iron and chromium hydroxides, which are then filtered and washed.
Why cant Sodium hydroxide not be used?
My thoughs are that hidroxides from Chromium start at ph 4-5 and you can easily go to ph 13+ with NaOh.
But with NaOH is easier to neutralize than with Sodium Carbonate (no fizzing and less quantity used)
Go SAFE, because stupidity and bad Luck exist.
|
|
|
bnull
National Hazard
   
Posts: 616
Registered: 15-1-2024
Location: Home
Member Is Offline
Mood: Slow
|
|
Chromium (III) hydroxide is soluble in sodium hydroxide.
|
|
|
RU_KLO
Hazard to Others
  
Posts: 248
Registered: 12-10-2022
Location: Argentina
Member Is Offline
|
|
this is allways true or ph dependant?
(I know that some hidroxides are soluble in NaOH excess)
For example if I keep the Ph below 9? (i consider NaOH in excess when Ph > 9 - more like 14+. or phenolftalein violet color.)
I know that with carbonate there is not such problem.
Go SAFE, because stupidity and bad Luck exist.
|
|
|
woelen
Super Administrator
        
Posts: 8083
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Cr(OH)3 only dissolves in fairly strong solutions of NaOH. If you have a solution with a pH equal to 9, then the concentration of NaOH only is 0.01
mmol/l, which is next to nothing. Cr(OH)3 certainly will not dissolve appreciably in that.
Even in a 100000 times stronger solution of 1 mol/l (which still is only moderately concentrated), I think that Cr(OH)3 will only dissolve with some
difficulty, but in such a solution, certainly some Cr(OH)3 will dissolve.
|
|
|
fx-991ex
Hazard to Others
  
Posts: 109
Registered: 20-5-2023
Member Is Offline
|
|
You need to oxidize the Cr(OH)3 to the chromate before acidifying to the dichromate.
Cr + HCl = CrCl3
CrCl3 + NaHCO3 = Cr(OH)3
Cr(OH)3 + NaClO = Na2CrO4
Na2CrO4 + HCl/H2SO4 = Na2CR2O7
Usually you react it with KCL to make potassium dichromate which is almost insoluble and will crash out.
|
|
|
RU_KLO
Hazard to Others
  
Posts: 248
Registered: 12-10-2022
Location: Argentina
Member Is Offline
|
|
Quote: Originally posted by fx-991ex  | You need to oxidize the Cr(OH)3 to the chromate before acidifying to the dichromate.
Cr + HCl = CrCl3
CrCl3 + NaHCO3 = Cr(OH)3
Cr(OH)3 + NaClO = Na2CrO4
Na2CrO4 + HCl/H2SO4 = Na2CR2O7
Usually you react it with KCL to make potassium dichromate which is almost insoluble and will crash out. |
Thanks, but Im trying to get the second step better (CrCl3 + NaHCO3 = Cr(OH)3)
instead of carbonate/bicarbonate by using NaOH. (Im not having good results with carbonate. The final chromate (Im after Potassium chromate) fizzles
when adding acid to it.. (probably because bad rinsing/cleaning the hydroxide with water)
Also for the oxidation step, was using H2O2 (30%), which after doing several times - and a lot of overflows (bubbling), readed that Iron + heat
decomposes the peroxide, so the oxidation is not good. (sorry for bad spelling) -I got the color change and all, but I think I could get more yield
with bleach. (or making first KClO)
Go SAFE, because stupidity and bad Luck exist.
|
|
|
Admagistr
Hazard to Others
  
Posts: 383
Registered: 4-11-2021
Location: Central Europe
Member Is Offline
Mood: The dreaming alchemist
|
|
Potassium dichromate is well known to be easily obtained very pure via recrystallization. So don't despair and then recrystallize it
From recrystallized very pure K2Cr2O7, very pure K2CrO4 can be easily prepared by adding a calculated amount of K2CO3 solution.
[Edited on 21-3-2024 by Admagistr]
|
|
|
bnull
National Hazard
   
Posts: 616
Registered: 15-1-2024
Location: Home
Member Is Offline
Mood: Slow
|
|
Maybe your SS has nickel in it. It would explain the black precipitate, the green/blue stuff.
(SS + HCl) -> green/blue solution + Sodium carbonate -> mixed iron/nickel/chromium hidroxides + oxidizer > filtrate: yellow solution >
removing water to cristallize.
It looks like nickel oxide to me. Ni3O4 is black, made from reacting nickel hydroxide (green) with hypochlorite (according to a
note in my notebook of 2008-2009). It was an excellent oxidiser (I still have a vial of it stored somewhere).
Quote: Originally posted by RU_KLO  | Got some green/blue precipitate, but it readly becomes rust brown upon adding H2O2 and heating)
Probably because Cr6+ instead of Cr3+??? |
Behaves like green rust. Was it a deep green, like broccoli? It could be either mixed iron hydroxides (the term is "layered hydroxides" or something)
or iron hydroxide and nickel hydroxide. The nickel hydroxide would still be green while the iron hydroxide oxidises and turns brown, and the overall
color seems a darker brown.
I suggest you test the yellow solution for iron and nickel. Sodium acetate gives dark orange to blood red with iron salts.
Another suggestion. You or someone else (I'm out of nickels salts) could try to reproduce the process "green/blue solution + Sodium carbonate ->
mixed iron/chromium hidroxides + oxidizer > filtrate: yellow solution" using metal salts as to give a proportion of Fe:Ni:Cr similar to some
stainless steel (the 304 (74:18:8), for example). If you get the same results, it is nickel.
[Edited on 24-3-2024 by bnull]
|
|
|
RU_KLO
Hazard to Others
  
Posts: 248
Registered: 12-10-2022
Location: Argentina
Member Is Offline
|
|
this weekend did a lot of tests.
First the hydroxides precipitation with NaOH went smoothly.
Taked an aliquote (10 ml) of the dark green chloride solution.
Roughly titrated with 1M NaOH (HCl concentration original used was 5,8M)
which gave me approx. 48ml 1M NaOH till Ph was around 7.
So for the rest (500ml dark green HCl metal solution) 500 ml 5M NaOH was used. The final Ph is around 6-7)
Currently is in the decanting, washing step.
waiting for final filtering and drying.
So next step (oxidation) is currently on investigation
So, I measured approx. 2grs of hydroxide paste (did not wait till dry) and prepared 3 samples (each sample with 2grs)
1) 18ml 20-30% H2O2 (it was originally 30%, but its 2 years old and I vented once every 2 months and some air escapes) + H2O till 30ml.
it was mixed, the color of the solution became brown. It was heated, bubbles were made. and at some point, they became violent and overflow in the
hotplate, becoming a nice cloud of steam, chromium and iron. Half was lost (15ml). Luckily it was not much. If this happened with 500 ml...
After decanting a light-yellow solution was found. very light yellow. Did not proceed further.
It seems that oxidizing with H2O2 is troublesome for me. 5 from 6 times, it overflows. Maybe it should be done cold, long time stirring and then
boiling off the excess peroxide.
I will not pursue this method.
2) 18ml of concentrated chlorine pool solution (do not know its concentration or composition) completed to 30ml with water was used.
got a strong yellow solution, Chlorine gas generation, very mild. Decanted, added a saturated KCl solution + little HCl to make it acidic.
Mix them together and reduce it by boiling and a lot (and I mean a lot) of salt was obtained (it was yellowish white, but it was not Chromate, because
when adding strong acid it did not become orange. Probably it was excess KCl or salts in the hypochlorite that precipitated, because I went from 60 ml
(30 ml original hypochlorite + 30 ml KCl solution) to 5 ml filtered yellowish orange solution - check left testube picture) - It was filtered 2 times,
once when the solution was on the 20ml mark, and the last when it was on 5ml.
Its not orange (and its acidic) - probably no or too diluted dichromate
Here are the questions:
1) how much Hypochlorite solution is needed to oxidize the hydroxide? I guess it depends on the concentration, but is there a visible end point, if
concentration is not known? I know it changes the color from brown to rust orange and some chlorine is made.
maybe when no more chlorine is produced.
Easy hypochlorite rough concentration titration?
and how is measured to a unknown hydroxide composition? (lets say 60% iron hydroxide 40% chromium hydroxide by weight)
I don’t know if this oxidation will be pursued, because obtained better results from the next one
3) oxidation with potassium persulfate.
Adapted this paper:
https://link.springer.com/article/10.1007/s42452-018-0020-0
18ml of a saturated potassium persulfate solution was made, completed to 30ml.
This was put inside an RBF used for distilling (the one that has an arm attached –cheap no glass joint
(example: https://encrypted-tbn0.gstatic.com/images?q=tbn:ANd9GcQqu_ZV...
As I do not own a fume hood, I use a funnel / scrubbing / aspirator, that is attached to the side arm. At the top, a cork with a thermometer and a
hole is used as a cap (this is to let air to get inside and aspirated by an aspirator made from a cheap water pump, an oxygen disposable washing
battle with water and it all ends in a 10 l compartment with NaOH. )
It was then heated and strirred (with magnet stirrer) to 70-80°C, the color changed from brown to rust orange. Then pushed a little more to 90C, it
became yellow-orange, and from re-reading the document, it indicates that H2SO4 is detrimental to Cr(VI) because it convert this back to Cr(III). So I
more or less panicked, remove the thermometer, tested for ph, it was red acidic.
(as a side note, when I removed the thermometer + cap I felt a stich in my eye, like a needle. I was using full cover eye protection, so probably SO2
or SO3 gas. Not much, because I did not smell it and was using 30 ml range) Next time OUTSIDE.
By fear I added a squirt (say 5 ml) of 6M KOH to the solution, turning from the yellow-orange to the rust orange + precipitation – Ph became dark
blue basic.
On reducing by boiling, a lot of salt precipitated – probably excess of persulfate.
Filtered when 5ml, and then added drops of 6M HCl. It turned orange. (check test tube right)
I will go with this method.
So questions:
1) Paper states optimize conditions: mass ratio of Na2S2O8 to Cr of 13.2 g/g,
How do I translate this to Potassium persulfate / gram weight of dry unknown hydroxide composition?.
Thinking:
Potassium persulfate: 270.322 g/mol
Iron(II) Hydroxide : 89.86 g/mol
Chromium (III) Hydroxide: 103 g/mol
Let’s assume 100g/mol hydroxides, so I already have 2,7 gr persulfate / 1gr hydroxide, so 5gr of persulfate / gram of Hydroxide?.
I think is too much, because used a saturated solution of persulfate (4.49 g/100 mL (20 °C) – 1,5gr in 30ml) for 2 grs of hydroxide and a lot of
salt – I think excess of persulfate or maybe is another salt) was obtained on boiling-reducing the liquid)
Will try half persulfate next time and also directing the SO3 (I think is SO3) gas to water to obtain H2SO4. Maybe this could be a way for
concentrating H2SO4....
2) The paper states: “The addition of sulfuric acid would increase the acidity of Cr(III) solution and increase the difficulty of oxidation of
Cr(III) to Cr(VI) [19]. The results shown in Fig. 5 indicated that the percentage of Cr(VI) decreased with the increasing of concentration of sulfuric
acid. In order to oxidize Cr(IIII) efficiently, the solution should be in low acidity condition or neutral condition.
Does H2SO4 reduces Cr(VI) to Cr(III)?
If you add sulfuric acid to chromate, you get dichromate.
Also, when the solution got to 90°C it changed colors from rust/orange to Yellow Orange, maybe dichromate was already be synthetized in one shot. (As
potassium per. was used, this could be a method to make potassium dichromate directly from Chromium Hydroxide)
(sorry for long post, long weekend)

Go SAFE, because stupidity and bad Luck exist.
|
|
|
RU_KLO
Hazard to Others
  
Posts: 248
Registered: 12-10-2022
Location: Argentina
Member Is Offline
|
|
Long post, but will try to explain everything that I made, so you can find where I screw up....
This weekend I tried the oxidation step.
1) hypochlorite
2) Potassium persulfate
1) Hypochlorite (used "concentrated pool chlorine" - which I think was Calcium hypochlorite
First I weighted in a test tube some random quantity of the Hidroxide paste (OH-paste), heated and when no more vapor was generated, reweighted.
Aprox 50% of the OH-paste was water.
So I took 32gr OH-paste --> used 16gr for calculations.(the idea of calculating, was not to use an excess of hypochloride, ie. not excess of salts)
but
probably I messed up, because the chemistry is not well known - for me.
Added 150 ml of the pool chlorine (I assumed 10% concentration) and dissolved (suspention) the OH Paste.
Heated with stirring at 80°C for an hour.
Then tried to filtrate it, first with a funnel with cotton, did not work, then with vacuum and a Buchner filter, worked halfway,
so last part was decanting.
An orange solution (somewhat turbid) was obtained. It was reduced from 200ml to 50ml when salts precipitated.
Next day it was filtrated, yellow salt was obtained. (it was tested with acid (HCl) to check color change to orange - did not change, so it was
discarded)
The filtrate - clean orange solution 50ml, it was acidic - was then heated and aprox 10 ml of 2M KCl solution was added.
When salts precipitated, it was filtered. The process was repeated 3 more times, heating, adding KCl and filtering.
The color of the salts afer each filtering process went from yellow white to yellow orange. (pic attached)
The salts from the 4 filtration were dried (heat) mixed together, approx 6.8grms were obtained.
It was redissolved in water (yellow-light orange solution) and KOH was added till strong basic (ph 14+) with ph paper. The idea was to transform
everything
to potassium.
Then I went back and forth with acid and hydroxide to check color change of precipitated salts.
Currently I have an orange solution (no precipitate) and some salts precipitation of a dark orange solution.
Will check in the future when some precipitate is formed.
Pros: hypochlorite is easily available.
Cons: a lot of salts precipitation, which are yellow, so indentifying the chromate/dichromate is difficult.
2) Potassium persulfate.
Performed the same operation as above, to check water content in the OH-Paste Aprox 20% of the OH-paste was water.
So 14,2grs of OH-Paste was calculated.
Prepared 100ml almost saturated at 20°C K2S2O8 (5grs/100ml) solution.
Place the OH paste and the solution in a boiling flask + thermometer, on a hot plate with stirrer.
target was 90C, but overshoot to 100°C, turn off heating and tried to mantain it in the 80-90° range.
After an hour, turned off the stirring, and let it decant.
Pippeted the solution (was clear brown) and reserved.
added another 100ml (5gr) K2S2O8, this time was more aware of the temp and let it another hour at 70-80°C.
decanted, pippeted the solution- it was clear brown.
Joined both solution - It was acidic.
An aliquote was taken and put in a test tube. added KOH, a lot of precipitate was found - probably Iron (III) hydroxide made from Iron sulfate (from
H2SO4 - made from the persulfate - and Fe(OH)2).
the upper part was a yellow solution. This was pippeted in another test tube, reduced by heating until some salt precipitated. tested with acid and
became light orange.
So probably Potassium Chromate + some other potassium sulfate / bisulfate)
Currently is decanting and then will be reduced by heating and cristallizing.
Pro: less salts, directly ussing potassium salts (K2S2O8/KOH) to get potassium salt
cons: potassium persulfate could be difficult to get and requires temperature control - not exceding 90°C (at 120° decomposes)
If someone could post a picture of a solution in a test tube of potassium chromate next to a solution of of potassium dichromate, for color checking,
it will be helpful for me.
(if possible, not saturated)
Thanks
   
Go SAFE, because stupidity and bad Luck exist.
|
|
|
bnull
National Hazard
   
Posts: 616
Registered: 15-1-2024
Location: Home
Member Is Offline
Mood: Slow
|
|
Find the MSDS of the hypochlorite you're using. Or search here: Productos para tratamiento de aguas (agua de piscinas) - Venta libre. What you have is most probably sodium hypochlorite. Calcium hypochlorite in
solution is not stable and decomposes in a few days, maybe hours. It's sold only as a granulated solid contaminated with calcium chloride. If you
can't find anything, try this instead: Determining the Percent Sodium Hypochlorite in Commercial Bleach.
The reactions you're interested in are
2CrCl3+3K2S2O8+14KOH→K2Cr2O7+6K2SO4+6KCl+7H2O
and
3NaClO−+2Cr3++4H2O→3Cl−+Cr2O2−7+8H+.
| Quote: | Does H2SO4 reduces Cr(VI) to Cr(III)?
If you add sulfuric acid to chromate, you get dichromate. |
No. The state of oxidation of chromium in both chromate and dichromate is +6. In solution, chromate and dichromate ions are in equilibrium, that
changes with the addition of an acid or base.
Anyway. We can rewrite the first equation above as
2Cr3++3S2O2−8+14OH−→Cr2O2−7+6SO2−4+7H++7OH−.
Cancel the superfluous OH- on both sides:
2Cr3++3S2O2−8+7OH−→Cr2O2−7+6SO2−4+7H+.
That's why they wrote that, "in order to oxidize Cr(III) efficiently, the solution should be in low acidity condition or neutral condition." Acids
favor the inverse reaction, which you don't want.
I'm not impressed with the paper. "In order to oxidize Cr(IIII) [sic] efficiently, the solution should be in low
acidity condition or neutral condition." The solution should have been made alkaline. Look at their equation. Holy flapping carp*. I know,
it would precipitate the hydroxide. But the precipitate will oxidise and dissolve as dichromate.
A 2002 paper treated a similar situation--in fact, it is closer to your situation than Peng's. Here it is: Fedoseev, A.M., Shilov, V.P., Budantseva, N.A. et al. Selective Recovery of Chromium from Precipitates Containing d Elements and Actinides: III.
Effect of S2O82-. Radiochemistry 44, 361–365 (2002).
*: I shouldn't be ranting here, I know. My apologies.
Look for a book on chemical ore assay. Treat the brown solid stuff as chromium-rich iron ore and perform the tests. You'll have a better idea of the
percentages and of how much hypochlorite or persulfate to use.
Use this page to find the quantities in moles or grams.
| Quote: | 1) Paper states optimize conditions: mass ratio of Na2S2O8 to Cr of 13.2 g/g,
How do I translate this to Potassium persulfate / gram weight of dry unknown hydroxide composition? |
There's no way. You need to know the composition of the hydroxide.
A smidgen (about the same mass as half grain of rice) of potassium dichromate in 1 mL of water, before and after the addition of saturated NaOH:

[Edited on 3-4-2024 by bnull]
|
|
|
RU_KLO
Hazard to Others
  
Posts: 248
Registered: 12-10-2022
Location: Argentina
Member Is Offline
|
|
thanks for the information and the pics.
It seems that I was using persulfate in excess.
for example the basic chromate was transformed in the acidic dichromate when the solution was heated (to reduce its volumen) meaning that on high
temperature (80-90C) the persulfate transformed into sulfuric acid, acidifying the solution....
So next step is to use less the persulfate trying to match 1,5 Cr(III): 1 S2O8 molarity. Difficult part is to know the quantity of Cr (III) in the
hidroxide paste...
and using a 2-3M KOH Solution,
[Edited on 5-4-2024 by RU_KLO]

Go SAFE, because stupidity and bad Luck exist.
|
|
|
bnull
National Hazard
   
Posts: 616
Registered: 15-1-2024
Location: Home
Member Is Offline
Mood: Slow
|
|
I found a way you can make an estimation. It's crappy as hell and quite longwinded. I don't know why I hadn't thought of it before. Here we go.
Things you'll need:
- hydrochloric acid;
- acetone (probably not needed; see Edit);
- ethanol;
- potassium or sodium hydroxide;
- three small (50 mL) beakers (A, B, C). Erlenmeyers are acceptable.
I assume you're in the stage after the convertion of metal salts to brown mixed hydroxides. Filter the slurry, wash the precipitate. Take about 2
grams of it and dry either in open air or over low heat (110 °C). After the brown stuff dried, take 1 gram to the empty beaker A.
Drip hydrochloric acid on the precipitate until all of it has dissolved. Put a couple of drops of hydrogen peroxide to convert all the iron to ferric
chloride. Heat the beaker to 160-180 °C to boil off the water and excess acid and peroxide, and to crystallise the salts. Let it cool to room
temperature, then add just enough water to dissolve everything.
Mix 5 mL of acetoneNote and 5 mL of ethanol (absolute if possible, 96.5° otherwise) in beaker B. Add 5 mL of mixed
solvent to the salts in beaker A and stir. This will dissolve iron(III) chloride (it's soluble in both acetone and ethanol) and
nickel(II) chloride (it's soluble in ethanol). Chromium (III) chloride is insoluble in both solvents. Transfer the solution to beaker
C. Add 2 mL of mixed solvent to the remaining salts in beaker A, stir, and transfer the solution to beaker
C. Repeat until you have used all the mixed solvent.
Edit: I've been thinking. It is quite obvious that, if you're extracting iron and nickel chlorides to leave them together in beaker
C, you won't need acetone. Both chlorides are soluble in ethanol. If you wanted to isolate the three metals, you would wash first
with enough acetone to remove iron, then with ethanol to remove nickel, keeping each metal in a different beaker. But that's not what you want. You're
interested in chromium, which will be in beaker A, and not B as I wrote before. Yes, I'm an ass.
Just use 10 mL (5+2+2+1) of ethanol to wash the crystals. What remains in beaker A is chromium(III) chloride. Or 100 mL (50+20+20+10)
and larger beakers for 10 g of dry brown powder if your scale measures only down to 0.1 g.
Now the fun part. Let the solvent in beaker A evaporate. Beaker A supposedly has all the chromium(III) chloride
while beaker C has all the iron(III) and nickel(II) chlorides. Add water to the salt in beaker A and a solution of
NaOH or KOH to convert the salt to chromium(III) hydroxide. Filter, wash the precipitate and let it dry.
Weigh the precipitate. This mass will correspond roughly to the percentage of chromium(III) hydroxide in the dry brown powder. Divide the mass by the
molar mass of Cr(OH)3 to find the number of moles of chromium(III) hydroxide per gram of brown powder. Add 10% to this number to account
for any losses.
The quantity of persulfate per gram of hydroxide paste depends on the water content of the paste and you have to adjust accordingly.
Note: The excess peroxide will be around tens of milligrams. It will either boil away or decompose in
the presence of transition metals.
[Edited on 7-4-2024 by bnull]
|
|
|
RU_KLO
Hazard to Others
  
Posts: 248
Registered: 12-10-2022
Location: Argentina
Member Is Offline
|
|
well still in the getting chromate/dichromate.
Here are pictures of the precipitates of the 2 process, Hypochlorite and Persulfate.
Nice filterpaper art.
The hypochlorite got some orange cristals - y need to separate them manually, the persulfate was orange when filtered, but then on standing became
white yellow and the filterpaper became light blue.
On the other hand, a new potassium hypochlorite was tried (first by making the hypochlorite -KOH + Cl2 - and then adding the hydroxide.
This was done yesterday, today got some cristals, so will give some days and then filter to check if K2Cr2O7 or KCl.
 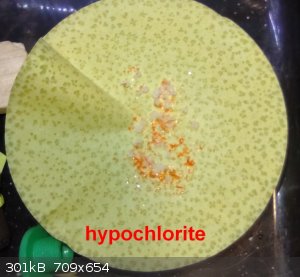 
[Edited on 19-4-2024 by RU_KLO]
Go SAFE, because stupidity and bad Luck exist.
|
|
|
RU_KLO
Hazard to Others
  
Posts: 248
Registered: 12-10-2022
Location: Argentina
Member Is Offline
|
|
Well to round up the experiment, got some Potassium Chromate- with some impurity.
Its green.
But when heated, first becomes yellow - when water is removed. Then red, and red dark blood - when liquid. When the liquid becomes solid, it regains
its bright yellow collor.
This yellow color keeps better than a sample, in which only water was removed (green - heat - yellow - hydration - green) and the returning green
color is more towards yellow than green.
Maybe the impuriry is volatile at the temp of liquid chromate.
Its Ok for my analytical chem. as chromium sample, precipitating agent.
Probably I could purify it by changing to dichromate, precipitation, etc, but the true is that the purification, evaporation, cristalization of
hexavalent chromium is not nice...
This are my 2 cents that I learned from this project.
1) try to start with Chromium hydroxide if possible
2) if your aim is the potassium salt, try to use all potassium reagents, for example potassium hypochlorite, potassim hydroxide, potassium carbonate.
With sodium -> bleach, Sodium hydroxide. If you mix them, it difficul to get nice separation of the cromate/dicromate potassium/sodium from the
oher potassium/sodium salts. All potassium and sodium salts are soluble and difficult to separate betweem them.
(example as homework:
prepare a 100ml solution containg 1M KCl + 1M NaCl + 1M KOH + 1M NaOH. Get the salts back.)
3) If you want chromate, try to get the dichromate first. Potassium dichromate is the best salt to cristallize. (Even is good as visual separation of
salts (also known as "Find the orange cristal in the cristal mountains..." game)
then convert it to the chromate with the corresponding hydroxide.
Almost freezing water can be used to remove other salt impurites from potassium dichromate cristals. (you will lose some, but its solibility at low
temperatures in low, compared to the other salts)
4) "All salts are yellow/orange" What I mean is that, although the solution and the salts that will precipitate during the cristalization course are
yellow or orange, does not mean that you have chromate or dichromate. Some chromate/dichromate tests needs to be done. (I used color change with
acid/base, but allmost evey salt cristallized passed, and lead cromate precipitation.)
Go SAFE, because stupidity and bad Luck exist.
|
|
|
|