Yttrium2
Perpetual Question Machine
    
Posts: 1104
Registered: 7-2-2015
Member Is Offline
|
|
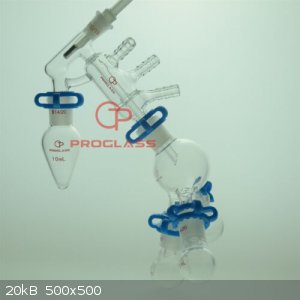
Micro Short Path - Extracting Essences
I have a few questions.
What would a flow chart diagram for the bench look like for this apparatus?
apparatus - T - Trap (What kind?) - Vacuum pump (What kind)
on the other side of the T is the manometer.
I know this is a smaller apparatus, so I'm guessing it would require a smaller type of vacuum. Now I know that the Dual Stage Rotary Vane Vacuum pump
is what I've heard mentioned being utilized a few times, but I do not know what other parameters to look for. Some cost thousands. I'm looking for
something budget minded. My application is below.
Could this 14/20 connector be used as a glass packed column?
Has anyone ever used a micro short path distillation apparatus?
Is it easy to get readings of the fractions coming off by seeing the thermometer? (the apparatus is so compact that I think the fractions could change
rapidly) -- I mean like normally a taller column allows for greater separation, -- could I separate within a degree with this glass?
what amendments or recommendations are there? is there a bleeder tube? It says on ebay that there is one, though, I do not see where it would go. Are
boiling stones needed??
This is a pretty small apparatus, others are around 2L-5L,
is there anything around 100mL for short path distillation flask setups?
I'm not sure if I were to use a larger pear with the system that it might overload the micro short path condenser.
And, at what exact point does the cow get turned, and is this a difficult task?
Is the risk of implosion something to be concerned about, or will the glass handle the vacuum? what vacuum wouldn't the micro short path apparatus
handle?
I'm wanting to get familiarized with doing fractional distillations, and distillations at reduced pressure. -- What are some neat projects I could
embark on to get the basics down, make some observations, and learn to use the equipment. - I think I would like to couple this apparatus kit with a
melting point apparatus, that way after doing vacuum distillations I may be able to take melting points of the substances to aid in my understanding.
|
|
|
Sulaiman
International Hazard
    
Posts: 3696
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
I think that you are considering extracting essences from plant sources,
the concentration of essential oils is sow low that a large volume of plant material is required to get a small quantity of oil.
Watch some YouTube videos.
separation of products with a 1oC difference in bp is difficult with a fractionating column,
impossible with a simple distillation.
When the Chinese manufacturer's of cheap glassware first released short path units
quite a few members here bought one
mine is in 'as new' condition 
[Edited on 21-5-2023 by Sulaiman]
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
Fery
International Hazard
    
Posts: 1016
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
I got 10 ml of oil from 1 kg of dry mint herb. I had to split the 1 kg of herb into halves and perform 2 distillations from 20 L still with 15 L space
for the herb where only half a kilo of mint herb fits (stems + leaves + flowers)
I have a cheap Chinese 2-stage rotary vane oil vacuum pump which reduces b.p. of 2-nitrotoluene from 220 to 70 C. So the pump is unsuitable for
distillation of anything boiling in range 150-200 C, for that I use water aspirator pump.
Even good column (= long enough, that means upto 1 m) with good packing (that means e.g. Raschig rings) and good insulation (vacuum jacketed) has
reduced efficiency at vacuum level due to reduced pressure of vapor. The efficiency depends on the exchange between upwards flow (vapor) and downwards
flow (condensate) - so a variable reflux ratio adjustable distillation head is necessary.
|
|
|
Texium
|
Thread Moved
23-5-2023 at 10:17 |
Sulaiman
International Hazard
    
Posts: 3696
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
Quote: Originally posted by Yttrium2  | | ... I'm wanting to get familiarized with doing fractional distillations, and distillations at reduced pressure. -- What are some neat projects I could
embark on to get the basics down, make some observations, and learn to use the equipment. - I think I would like to couple this apparatus kit with a
melting point apparatus, that way after doing vacuum distillations I may be able to take melting points of the substances to aid in my
understanding... |
I usually test a new setup by distilling water.
This tests that everything is working and 'calibrates' my thermometer(s)
It also let's me get an idea of time required for a real experiment,
quantities of ice used etc.
If the glassware is large then I'd probably make some usable distilled water.
In my experience so far;
Simple distillations at atmospheric pressure are the place to start,
then fractional distillations at atmospheric pressure,
That's where the bullk of my experience is.
I did a quick test of simple distillation at reduced pressure,
Then realised that fractional distillation at reduced pressure is possible,
but not practical without quite a lot of work and equipment...
... significant commitment to a project.
Fractional distillation works best when you can't see it due to stuff like thermal insulation,
If you want to look at it you disturb equilibrium,
So you will hardly see your glassware or any reaction,
which largely defeats the purpose of glassware.
An easy to use melting point measurement device is on my wish list.
My analytical chemistry skills are pathetic - a continuous source of annoyance.
Verifying the products of my experiments is a whole huge area in itself,
almost always harder to test than to make 
PS if anyone wants to donate a gcs/ms setup, uv-vis spectroscope, electron microscope etc. that would be nice 
Meanwhile it's flame tests, indicators and wet chemistry.
And I'm still only doing 'simple' chemistry.!
My admiration of earlier alchemists/philosophers/chemists keeps growing.
Luckily there is enough weird/fun/fascinating stuff to keep me interested in some chemistry.
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
|