Organikum
resurrected
    
Posts: 2337
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: frustrated
|
|
Knick ph-meter Type 35
Well I just could not resist for 15 € and bought me a Knick Type 35 ph-meter.
Naturally I would love to have it functional and more I would want to know HOW to use the darn thing.
I am aware of the basic functioning of it, measuring mV of the elctrode what resembles ph taking into account the drift due to temperature. What I do
not know though is the function of:
left side:
- Stufen Komp(ensation) whats stepwise compensation translated but what does it mean?
right side:
- left is titration, right is DS. Whats DS? Two channels, to every chanel belongs an electrode, connectors for two kinds are present. I can choose one
channel and then choose between titration - nada - measuring, or DS-nada-measuring. I suspect that DS is just normal ph -meter and titration is
somehow dynamic following a changing ph when titrating. The bottom knob labeled titrationcan be turned left or right and does what? Why are the small
pre-select buttons labeled 54/56/58 ?
Middle position of top button is Eich(ung) say calibration. I guess the two connectors on top in the middle are for a Calomel electrode and well thats
easy then.
Delta ph and delta E will be adjustment of the logarithmic function.
So how to do a titration with this? If somebody knows, I would be really happy, I searched the net and mailed the company but nothing. Or if somebody
knows a old book online where they explain the general use of those oldies.
It weighs 7 kg btw. Unbelievable heavy.
Pictures attached




[Edited on 22-3-2023 by Organikum]

|
|
|
Jeeves225
Harmless

Posts: 21
Registered: 16-2-2023
Member Is Offline
Mood: Salty
|
|
Nice thing! Surprisingly little information about it though on the web.
|
|
|
Rainwater
National Hazard
   
Posts: 919
Registered: 22-12-2021
Member Is Offline
Mood: indisposition to activity
|
|
Its got tubes. Older than the web
"You can't do that" - challenge accepted
|
|
|
Cathoderay
Hazard to Self
 
Posts: 54
Registered: 29-1-2023
Location: US-Texas
Member Is Offline
|
|
My background is in electronics. I also restore vintage electronics.
Tubes do wear out of course, but the other components in this instrument are higher quality than in a typical consumer item. My guess at this point is
that it dates from about 1980.
Do you know about titration using an indicator solution? A meter will not make it any easier, but it could make it more precise.
You would need a pH probe and to adjust it a couple of precise buffer solutions like pH 4.0 and pH 7.0. I wouldn't turn the unit on without a probe at
this stage, you couldn't learn much about the unit if you did.
The input terminals of a pH meter have a very high impedance. A typical digital voltmeter would have an input impedance (load it puts on whatever is
measured) of about 10,000,000 ohms. A pH meter would have an impedance about a thousand times higher. That makes a pH meter somewhat delicate
electrically. The electrical insulation has to be very good, many times glass and Teflon are used around the input circuit. Even the contamination
from your figures can effect the operation.
Unfortunately I cannot read German very well so I can't help you with that.
The best thing is to find a manual on that meter or a similar meter.
"In for a penny, in for a pound."
|
|
|
Organikum
resurrected
    
Posts: 2337
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: frustrated
|
|
Thanks. The device has a dedicated calibration setting, I believe titration means something different. The elctrodes have not changed, only the
connectors so I got me two cheap ones off Amazon and replaced the coaxial connectors with some banana plug connectors which fit perfectly. I also got
me the ceramic AC connector. HHave yet to switch on though....
I doubt it is younger then 1960's, I looked for Knick phh meters on the net and they follow very accurately the design taste of the periods, so 80's
would look more like (fake) digital, very square without any rounded corners.
There are plenty of those devices on the market and for very cheap too. I might keep this just for decoration and get me another one with an output
for a writer, the signals easily converted to be useable for data logging or even automatic ph adjustment.
|
|
|
Cathoderay
Hazard to Self
 
Posts: 54
Registered: 29-1-2023
Location: US-Texas
Member Is Offline
|
|
Actually I was leaning toward '60s to '70s judging from the tubes.
I'm not an expert on chemistry but titration, I think, always involves slowly adding one solution with a known concentration of one chemical to one
with an unknown amount of another chemical so that they react and produce a neutral pH (7). At the point where a pH of 7 is reached (end point), from
the volume of the known solution used it is possible to calculate the amount of the unknown chemical. One way to know when the end point is reached is
with a indicator dye that changes color with pH. The other way is with a pH meter.
It could very well be that this meter has a mode where the sensitivity is extra high near a pH of 7 for titration use.
pH probes very a bit from unit to unit so the meter has to be calibrated to the probe. There voltage can also vary a bit with temperature.
I have a small digital pH meter and it has a temperature knob and a calibrate knob. It came with small bottles of 4.0 and 7.0 buffers. The probes
always have to be kept wet.
https://en.wikipedia.org/wiki/PH_meter
|
|
|
Texium
Administrator
       
Posts: 4580
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
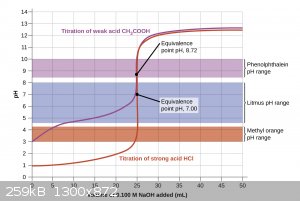
The endpoint will not always be 7, it depends on what you are titrating. A strong acid/strong base titration will technically have an endpoint at
exactly 7, but the pH will also rapidly change as soon as that endpoint is reached. For titrating a weak acid with a strong base, though, the endpoint
will be when the pH = the pKb of the salt that is being formed. For example, if you titrate acetic acid with sodium hydroxide the endpoint will be
9.25 because sodium acetate itself is a weak base. The same goes for a weak base with a strong acid, just in the opposite direction. This is why
indicators with different transition ranges are used and there’s not one that works for every system.
|
|
|