DrDevice
Hazard to Self
 
Posts: 75
Registered: 19-3-2012
Member Is Offline
Mood: Incompatible with carbon based lifeforms
|
|
Fischer synthesis of methyl 3,4,5-trimethoxycinnamate
A straightforward Fischer methyl esterifcation of 3,4,5-trimethoxycinnamic acid.
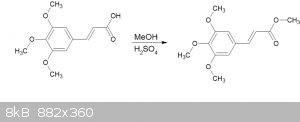
The cinnamic acid was synthesized by the Knoevenagel method reported here:
https://www.sciencemadness.org/whisper/viewthread.php?tid=159181
2ml of conc. H2SO4 was added to 50ml anhydrous methanol.
4.73g (19.8mmol) of 3,4,5-trimethoxycinnamic acid (3,4,5-TMCA) was dissolved to the resulting mixture (it dissolved quite readily), and refluxed for
18 hours.
After 18 hours, the solution was allowed to cool to RT, after which crystals of the methyl ester precipitated.
Over a few trials, I tried a couple of different approaches at this point. If the solution is placed in the freezer, quite a respectable yield is
obtained after several hours without further work.
Alternatively, the liquid remaining after filtering off the precipitate can be returned to reflux. The solids are washed with a little warm MeOH with
the "washings" added to the filtrate.
I presume that removing the desired product is as effective as removing water from the reaction solution for driving it to completion (??)
The filtrate is then refluxed for a further 8 hours. In the last stages of the reflux, I removed the reflux condenser to allow most of the MeOH to
evaporate.
After cooling to RT, a saturated NaHCO3 solution and EtOAC was added, and the organic layer separated. As second extraction of the aqueous layer was
also performed.
The combined organic extracts were washed with brine and dried with MgSO4, and then the mixture was filtered and solvent evaporated.
The solids from the original precipitation (2.68g) and following from the second reflux and extraction (1.90g) were combined and recrystalized from a
4:1 MeOH:water mixture. The crystals were washed with water during vacuum filtration.
3.99g (15.8mmol) of crystalline material was recovered. This equates to a 80% yield.
MP 96 - 97 (lit 99 - 100).
TLC with 5:5:1 toluene:acetic acid:ethanol gave a Rf of 0.75 (single spot), compared to an Rf of 0.59 for the TMCA.
Notes:
The literature provides a couple of similar procedures, but they seems to go for an enormously large excess of MeOH.
I was surprised that the methyl ester was much less soluble in the MeOH than the 3,4,5-TMCA. I had originally anticipated pausing the reflux at the 18
hours mark, cooling, drying the mixture, then returning to reflux for several more hours, then extracting.
Possibly at 18 hours the reaction is "complete" and just leaving the solution in the freezer overnight, filtering, and extracting from the solution
would give the same result.
For recrystalization, straight MeOH also works, and much bigger crystals are obtained, but a fair bit of product is retained in the solvent.
[Edited on 26-1-2023 by DrDevice]
[Edited on 26-1-2023 by DrDevice]
|
|
|
Fery
International Hazard
    
Posts: 1026
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
Excellent yield !!! And even at this small scale experiment !!!
After 18 hours the reaction is at equilibrium. If you want reaction to complete, all the water should be removed - Dean-Stark trap apparatus +
entrainer (hexane, cyclohexane, heptane, benzene, toluene, xylene). Using Dean-Stark the reaction could be complete in <1 hour.
|
|
|
DrDevice
Hazard to Self
 
Posts: 75
Registered: 19-3-2012
Member Is Offline
Mood: Incompatible with carbon based lifeforms
|
|
I don't have a "real" Dean-Stark trap to work with, I guess I could bodge one up, but they aren't too expensive so I might just buy one.
Looking at azeotrope data, for the entrainers you mentioned, only hexane/water has a boiling point lower than methanol. What proportions
methanol/hexane would you suggest?
|
|
|
arkoma
Redneck Overlord
      
Posts: 1763
Registered: 3-2-2014
Location: On a Big Blue Marble hurtling through space
Member Is Offline
Mood: украї́нська
|
|
a dean stark can be rigged up if you put the tip of the 105 takeoff in your boiling flask, and change out the thermometer with a condensor, and a sep
funnel on bottom of disty head. forgive my terrible drawing skills!

"We believe the knowledge and cultural heritage of mankind should be accessible to all people around the world, regardless of their wealth, social
status, nationality, citizenship, etc" z-lib
|
|
|
Fery
International Hazard
    
Posts: 1026
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
I use 10 ml Dean Stark, it has extra cca 5 ml space above graduation (so totally about 15 ml). So I use cca 25 ml of hexane for 0,5 mol ester
synthesis. After assembling everything, I fill the trap from top using a funnel and then add extra 10 ml of hexane through the trap so that 10 ml
flows into reaction. There should be more hexane than the volume of the trap. At 0,5 mol scale the trap is filled with water at the end of reaction so
then there is more hexane in the reaction at the end (cca 20 ml) than at the beginning (cca 10 ml). At smaller scale there will be very tiny amount of
water trapped. I did not see such small traps but perhaps 1 ml trap would be enough for your small scale. Or you can at least fill bigger trap with
small glass balls or glass rods to reduce its volume??? If you can buy then as smallest as possible - would be best for your scale and if doing bigger
scale you can sometimes (e.g. every 5 minutes) drain the water from small trap. The smallest I have ever seen on ebay had 10 ml (plus some dead volume
above graduation). Btw I use medicinal petrolether b.p. 60-68 C which is hydrogenated fraction of isohexanes (you should check content, there must NOT
be unsaturated hydrocarbons - they could hydrate into unwanted alcohols and esters), it is very cheap here, readily available and works OK, just the
temp. of vapor is quite low (maybe 50 C) so you have to heat slowly at 0,5 mol scale or use efficient condenser as there is a lot of isohexanes vapor
for a little of water.
If you plane to synthesize a lot of esters, just buy it on ebay. But at your scale without trap and 80% yield you can improve only a little, so
consider whether it is worth of it. I synthesized >20 esters so for me it was necessary and saved me a lot of days as every esterification was done
in 1 hour.
|
|
|
DrDevice
Hazard to Self
 
Posts: 75
Registered: 19-3-2012
Member Is Offline
Mood: Incompatible with carbon based lifeforms
|
|
Well, this Dean-Stark approach is pretty bloody good :-)
I acquired a suitable piece of glassware. The "water receiver" was a bit too large for my scale of reaction, so I loaded it with some glass tube and
glass beads, then almost filled it with hexane (thanks for the suggestion, Fery).
I used 3.2g of 3,4,5-trimethoxy cinnamic acid, 30ml of MeOH, 2ml of sulfuric acid and 25ml of hexane (plus whatever hexane was loaded in the water
receiver).
After several minutes of reflux I could see tiny amounts of denser liquid drippling into the receiver. Seems like it wasn't just water, but some
methanol was coming over also (I tried putting salt in the liquid - it didn't dissolve). I drained about 4ml of methanol/water from the trap at 30
minutes, and another 3ml at the end.
I stopped the reaction after 1 hour. There was copious solid precipitate. I allowed the reaction mixture to cool to RT then I placed it in the fridge.
The solution was filtered and solids washed with water, and then the solids were recrystalized with 4:1 MeOH:water as before.
Yield was 81%, MP of 96 - 97C as before. So not much difference in yield, but the time saving was remarkable.
|
|
|
|