Rainwater
National Hazard
   
Posts: 919
Registered: 22-12-2021
Member Is Offline
Mood: indisposition to activity
|
|
Measuring azeotropes
There are a lot of questions here about what mixtures form azeotropes.
I haven't found anything about testing for azeotropes.
Would it be possible to determine an azeotrope by measuring the boiling point of a solution across the 0~100% concentration range
Say with something like this
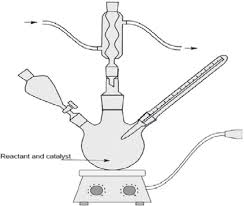
(Image stolen from here)
Edit: added procedure for clarity
Step 1) boil a solution containing 100% "A" and 0% "B". Record temp
Step 2) increase "B" concentration and record temp
Step 3) repeat until a 50/50 mixutre is obtained
Step 4) boil a solution containing 0% "A" and 100% "B"
Step 5) increase "A" consentration and record temp
Step 6) repeat until a 50/50 mixture is obtained.
[Edited on 30-12-2022 by Rainwater]
"You can't do that" - challenge accepted
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
When you boil a mixture that is not the azeotrope mixture you will always hit all three temperatures; the two seperate boiling points and that of the
azeotrope. The question is whether your separation is good enough to be able to measure them.
|
|
|
Rainwater
National Hazard
   
Posts: 919
Registered: 22-12-2021
Member Is Offline
Mood: indisposition to activity
|
|
In this example, I'm not trying to separate anything.
There is no collection, just 100% reflux so the volume of liquid does not change, and a thermometer.
I noticed a trend in some boiling point charts, for example
This one about H2SO4 and water.
As the concentration of sulfuric acid increases from 0% the boiling point also increases.
But there comes a point where the increase in concentration results in, the change in the boiling point reversing.
This is also the azeotrope for this acid.
Another example is with HNO3
And it shows the same trend.
Again with ethanol same thing.
As the concentration of ethanol increases, the BP decreases, until the azeotrope is reached, then the change in BP reverses.
"You can't do that" - challenge accepted
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
The difference in boiling temperature happens depending on the concentration, so if you want to see a change you will have to get a difference in
concentration right? In order to do so you will have to separate.
At 100% reflux you will just measure the boiling point of the mixture.
Edit: ah, I see you just want to measure the boiling point of the mixture. Yes, that should work. But wouldn't it be easier to boil away one of the
components until you reach the azeotrope and then measure the concentration of each?
[Edited on 30-12-2022 by Tsjerk]
|
|
|
Rainwater
National Hazard
   
Posts: 919
Registered: 22-12-2021
Member Is Offline
Mood: indisposition to activity
|
|
Quote: Originally posted by Tsjerk  |
But wouldn't it be easier to boil away one of the components until you reach the azeotrope and then measure the concentration of each?
|
Honestly, I'm not sure. That's why I'm asking.
I want to start experimenting with TLC places and try to run a column.
I'm debated on the difficulty of extracting solvents from local sources vs purchasing.
I've found a few which contain pure solvents like
Tetrachloroethylene, acetone, and basically what the hardware store sells.
Stuff that's ready to use.
When comparing msds sheets to known azeotropes, there is a lot of missing information.
I want to fill in the blanks so I can better source small quantities as needed.
Edit: fix spelling
[Edited on 31-12-2022 by Rainwater]
"You can't do that" - challenge accepted
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Quote: Originally posted by Tsjerk  | | When you boil a mixture that is not the azeotrope mixture you will always hit all three temperatures; the two seperate boiling points and that of the
azeotrope. The question is whether your separation is good enough to be able to measure them. |
This is not true.
Phase diagrams give you your answer. But I am not about to give a full explanation on NY Eve. Too much other celebrating to do.
|
|
|
Sulaiman
International Hazard
    
Posts: 3692
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
Most of the common azeotropes are listed here
https://en.wikipedia.org/wiki/Azeotrope_tables
Binary and tertiary.
For more info scroll down to References.
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Quote: Originally posted by j_sum1  | Quote: Originally posted by Tsjerk  | | When you boil a mixture that is not the azeotrope mixture you will always hit all three temperatures; the two seperate boiling points and that of the
azeotrope. The question is whether your separation is good enough to be able to measure them. |
This is not true.
Phase diagrams give you your answer. But I am not about to give a full explanation on NY Eve. Too much other celebrating to do.
|
Uh, no, of course not. Otherwise you could just distill dilute ethanol to absolute. You will only reach the boiling point of one of the components
when it is pure and you will never get one of the components pure because of the azeotrope. I don't know what i was thinking yesterday.
|
|
|
Rainwater
National Hazard
   
Posts: 919
Registered: 22-12-2021
Member Is Offline
Mood: indisposition to activity
|
|
Its the ethanol. It has that effect on me too. And this fourm helps me think my problems out
The heart of the question is,
how can I use experimentation to draw a "liquid vapor diagram"?
What is the procedure for making the diagram?
To me it seams like a simple algebra problem.
Once I have a concentration vs boiling point chart at a given pressure.
It appears possible to use simple distillation to determine the vapor concentration mathematically.
By measuring the volume of distillate over a given time frame,
and recording the starting and ending boiling temperature for that fraction, the average concentration of the boiling flask will be known, then do the
math and calculate the difference in concentration vs volume of the boiling flask to determine what the vapor concentration is.
Crap I can't put these thoughts into words. Let me do it by example.
.
Let's say this was observed with a mythical solution,
I've already composed the boiling point/concentration chart.
In an ideal world.
With only 1 liquid vapor cycle. 1 theoretical plate.
| Code: |
100ml of a 50% mixture. 50ml of "A", 50ml of "B" are in the boiling flask.
compound "A" in the boiling flask increased to 55% during this fraction of 10ml
0.5ml of "A" and 9.5ml of "B" are in the distillate.
this give the distillate a 5% concentration of "A", and 95% concentration of "B"
90 ml of a 55% mixture. 49.5ml of "A", 40.5ml of "B" are in the boiling flask.
compound "A" in the boiling flask increased to 61% during this fraction of 10ml
0.7ml of "A" and 9.3ml of "B" are in the distillate.
this give the distillate a 7% concentration of "A", and 93% concentration of "B"
80ml of a 61% mixture. 48.8ml of "A", 31.2ml of "B" are in the boiling flask.
compound "A" in the boiling flask increased to 67% during this fraction of 10ml
1.9ml of "A" and 8.1ml of "B" are in the distillate.
this give the distillate a 19% concentration of "A", and 81% concentration of "B"
|
"You can't do that" - challenge accepted
|
|
|