| Pages:
1
2
3
4 |
sceptic
Harmless

Posts: 49
Registered: 7-6-2022
Location: Southern Africa
Member Is Offline
|
|
AlCl3 by double displacement?
I've recently been looking for an easy way to get aluminium chloride, and I came up with this idea. I don't know whether it would really work, but
I've tried an experiment, and it seemed to work. If anyone's heard of this reaction before, or knows that it definitely won't work, please let me
know.
Theory:
A mixture of anhydrous alum (potassium aluminium sulfate) and sodium chloride should undergo a double displacement reaction when heated, forming
potassium sulfate, sodium sulfate, and aluminium chloride, which sublimates at 180 Celsius and separates from the mixture.
$$2KAl(SO_4)_2(s) + 6NaCl(s) \longrightarrow K_2SO_4(s) + 3Na_2SO_4(s) + 2AlCl_3(g)$$
Experiment:
I took 1.5 grams of powdered burnt (anhydrous) alum and mixed it thoroughly with 1.0 grams of dried, powdered table salt in a test tube, which I then
sealed. The next day, I put a wooden cork with a hole through it in the end of the test tube, and put a balloon over the other end of the cork, in
order to allow the air inside the test tube to expand. I heated the bottom end of the test tube in the flame of an alcohol burner. After heating it
for 1-2 minutes, I noticed that droplets of water had formed near the unheated end of the test tube. I removed the test tube from the flame, opened
it, and wiped the droplets out with a paper towel. I put the end in the flame again. After several minutes, I saw that a few small droplets of water
had formed near the unheated end, and that what looked like a thin white mist of particles had formed on the glass inside the test tube about an inch
from the mixture at the bottom. This layer was much closer to the heat than the water droplets were. The layer cleared when I moved it toward the
flame, and reformed when I removed it. I also saw that the mixture at the hot end turned a yellow, then light orange colour where it was heated most
strongly, and seemed to turn white again when the heating was removed. After several minutes, I saw no more changes, and the white layer didn't seem
to get any thicker.
I removed the test tube from the heat, opened it, and added a few drops of water. I held a piece of pH paper in the steam that rose, and it turned
dark red. I would expect this to mean that aluminium chloride was present, that it hydrolysed to aluminium hydroxide and hydrogen chloride. I assume
that the white layer was aluminium chloride. The low yield seems reasonable, since the reaction would only occur where the alum and salt particles
were touching.
Questions:
Is this reaction even possible? I've never heard of it before.
If this reaction isn't possible, what would have caused the acidic vapors?
What would have caused the yellow/orange color in the heated part of the test tube?
[Edited on 19-11-2022 by sceptic]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Per se it's not a bad idea because AlCl3 is volatile, so it leaves the reagent mix, thus moving the equilibrium to the right (Le Chatelier Principle).
I had good success with something similar:
$$3\text{ZnCl}_2(anh,s)+2\text{Al}(s)\longrightarrow 3\text{Zn}(s)+2\text{AlCl}3(g)$$
This too works because the AlCl3 leaves the party.
Details are reported somewhere on this site by me. Search and ye shall find.
----------------------
I think at the very least an alcohol burner will not deliver the temperature you need.
And I think your alum/sodium chloride is off.
[Edited on 19-11-2022 by blogfast25]
|
|
|
sceptic
Harmless

Posts: 49
Registered: 7-6-2022
Location: Southern Africa
Member Is Offline
|
|
I've seen your preparation here, it's what gave me the idea  . However, I don't
have access to anhydrous zinc chloride, and it seems no easier to make than aluminium chloride. It seems from what I've found that alum and aluminium
sulfate don't significantly hydrolyse when they're heated to dehydration. If this works, it means that aluminium chloride could be made from readily
available materials. . However, I don't
have access to anhydrous zinc chloride, and it seems no easier to make than aluminium chloride. It seems from what I've found that alum and aluminium
sulfate don't significantly hydrolyse when they're heated to dehydration. If this works, it means that aluminium chloride could be made from readily
available materials.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2786
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Every time this discussion comes up I point out that MnCl2 can be dried by heating and should react just fine with Al. So I'll do it again. I don't
have the equipment to try it but I do have a keyboard.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by clearly_not_atara  | | Every time this discussion comes up I point out that MnCl2 can be dried by heating and should react just fine with Al. So I'll do it again. I don't
have the equipment to try it but I do have a keyboard. |
There are other chlorides that would work: CuCl2, PbCl2. Maybe even NaCl or CaCl2.
Are you sure MnCl2.2H2O won't hydrolyse or oxidise? I once decarbonated MnCO3 to obtain MnO and had to use an inertial blanket to prevent oxidation to
+3/+4.
[Edited on 19-11-2022 by blogfast25]
|
|
|
Rainwater
National Hazard
   
Posts: 919
Registered: 22-12-2021
Member Is Offline
Mood: indisposition to activity
|
|
Feel free to double check the math but looks like you need more heat.
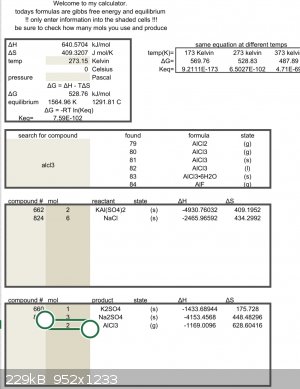
Edit:
[Edited on 20-11-2022 by Rainwater]
"You can't do that" - challenge accepted
|
|
|
DraconicAcid
International Hazard
    
Posts: 4332
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
I have no direct experience or citation to back this up, but I suspect you'd be more likely to decompose the sulphate than to get AlCl3.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2786
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
CuCl2 would have an excessively high reaction energy; it's been tried with concerning results. PbCl2 would potentially release lead vapor (10 Pa at
715 C), which is bad. NaCl or CaCl2 will not react, see: https://www.drjez.com/uco/ChemTools/Standard%20Thermodynamic...
Quote: Originally posted by blogfast25  |
Are you sure MnCl2.2H2O won't hydrolyse or oxidise? I once decarbonated MnCO3 to obtain MnO and had to use an inertial blanket to prevent oxidation to
+3/+4.
|
Gotta admit I'm going off Wikipedia here, which says the dihydrate dehydrates at 135 C. But I did find a paper showing that manganese (II) malonate
dehydrates at 180 C before being oxidized when the malonate anion decomposes around 350 C (attached).
EDIT: Castor and Basolo report that "The stronger metal-chloride bonds deduced for the analogous manganese and nickel [hydrate] complexes favor
decomposition to anhydrous chlorides as observed." [brackets mine] I have to admit I'm a little surprised because I thought that NiCl2*xH2O hydrolysed
on heating. (attached)
EDIT2: You did this reaction! Apparently NH4Cl is required. Still easier than ZnCl2.
http://www.sciencemadness.org/talk/viewthread.php?tid=10847
| Quote: | | Feel free to double check the math but looks like you need more heat. |
For a gas evolution reaction you don't need to reach equilibrium; if Keq is not extremely low you'll see some gas evolution. This is because the
entropy of a gas is measured at atmospheric pressure, but much higher at low partial pressure. With that said, it's not clear that he would have
gotten there.
| Quote: |
Attachment: mohamed1994.pdf (783kB)
This file has been downloaded 235 times
Attachment: castor1953.pdf (537kB)
This file has been downloaded 254 times
[Edited on 20-11-2022 by clearly_not_atara] |
|
|
|
sceptic
Harmless

Posts: 49
Registered: 7-6-2022
Location: Southern Africa
Member Is Offline
|
|
Thanks for the information! I tried to do those calculations, but I wasn't able to find the entropies of formation for the various sulfates. What
calculator are you using?
| Quote: |
I have no direct experience or citation to back this up, but I suspect you'd be more likely to decompose the sulphate than to get AlCl3.
|
It did occur to me that if the sulfate was somehow being reduced to sodium sulfide, that would explain the yellow color. However, I can't think of
anything that would be acting as a reducing agent.
| Quote: |
Every time this discussion comes up I point out that MnCl2 can be dried by heating and should react just fine with Al. So I'll do it again. I don't
have the equipment to try it but I do have a keyboard.
|
Do you know at approximately what temperature that would react? I might try that, but it would be difficult to make an apparatus to contain and
capture the fumes from a thermite-like reaction.
|
|
|
Rainwater
National Hazard
   
Posts: 919
Registered: 22-12-2021
Member Is Offline
Mood: indisposition to activity
|
|
https://www.sciencemadness.org/whisper/viewthread.php?tid=15...
Quote: Originally posted by sceptic  |
| Quote: |
Every time this discussion comes up I point out that MnCl2 can be dried by heating and should react just fine with Al.
|
Do you know at approximately what temperature that would react?
|
I have never performed this reaction, but have been using that excel sheet with great success to do other reactions.
Delta g = zero at 604c
but this is only an estimate so hotter than that. The reagents might need to be a liquid before the reaction will start.
Aluminum melts just above that temperature.
You will have to try it to find out.
Test tube and alcohol lamp will easily reach that point.
[Edited on 20-11-2022 by Rainwater]
"You can't do that" - challenge accepted
|
|
|
sceptic
Harmless

Posts: 49
Registered: 7-6-2022
Location: Southern Africa
Member Is Offline
|
|
Quote: Originally posted by Rainwater  |
Delta g = zero at 604c
but this is only an estimate so hotter than that. The reagents might need to be a liquid before the reaction will start.
Aluminum melts just above that temperature.
You will have to try it to find out.
Test tube and alcohol lamp will easily reach that point.
[Edited on 20-11-2022 by Rainwater] |
I probably could reach that temperature, but I don't think my test tube would survive it. According to this source, borosilicate glass begins to deform at 525 Celsius, and shouldn't be heated above 300 Celsius. However, since aluminium chloride is
being continuously removed from the products by sublimation, it would drive the the reaction even below that temperature, so it might not be necessary
to heat it that much. At least it doesn't look like it would be excessively exothermic, but I would still be hesitant to try it in a test tube.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2786
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
The reaction is endothermic. Oxidation of MnCl2 by atmospheric oxygen might drive the result in some heating.
In the solid phase, the reaction is only very slightly endothermic, deltaH = 37 kJ/mol. I wonder if it could be driven by the solvation enthalpy of
AlCl3 in some solvent (like ether) that can be removed by heating.
[Edited on 20-11-2022 by clearly_not_atara]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by clearly_not_atara  |
EDIT2: You did this reaction! Apparently NH4Cl is required. Still easier than ZnCl2.
http://www.sciencemadness.org/talk/viewthread.php?tid=10847
| Quote: | | Feel free to double check the math but looks like you need more heat. |
Yes, I remember it now. Using NH4Cl is a trick because its vapour is partly dissociated into HCl + NH3. The former then protects the chloride.
I used this also on NdCl3.
ZnCl2 anh. is cheap and readily available. Very useful for chlorinating alcohols. But buying MnCl2 anh. would be more difficult.
Has anyone tried MnCl2 + Al?
[Edited on 20-11-2022 by blogfast25] |
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by sceptic  | I put the end in the flame again. After several minutes, I saw that a few small droplets of water had formed near the unheated end, and that what
looked like a thin white mist of particles had formed on the glass inside the test tube about an inch from the mixture at the bottom. This layer was
much closer to the heat than the water droplets were. The layer cleared when I moved it toward the flame, and reformed when I removed it. I also saw
that the mixture at the hot end turned a yellow, then light orange colour where it was heated most strongly, and seemed to turn white again when the
heating was removed. After several minutes, I saw no more changes, and the white layer didn't seem to get any thicker.
I removed the test tube from the heat, opened it, and added a few drops of water. I held a piece of pH paper in the steam that rose, and it turned
dark red. I would expect this to mean that aluminium chloride was present, that it hydrolysed to aluminium hydroxide and hydrogen chloride. I assume
that the white layer was aluminium chloride. The low yield seems reasonable, since the reaction would only occur where the alum and salt particles
were touching.
|
Acc. Wiki, Al sulphate decomposes to alumina and SO3 between 580 and 900 C. So maybe the white layer was SO3?
https://en.wikipedia.org/wiki/Aluminium_sulfate#Chemical_rea...
|
|
|
Rainwater
National Hazard
   
Posts: 919
Registered: 22-12-2021
Member Is Offline
Mood: indisposition to activity
|
|
https://www.chemedx.org/JCESoft/jcesoftSubscriber/CCA/CCA3/M...
"You can't do that" - challenge accepted
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
That's info you can find in Wiki but here we're trying to avoid Cl2 (or HCl).
|
|
|
Rainwater
National Hazard
   
Posts: 919
Registered: 22-12-2021
Member Is Offline
Mood: indisposition to activity
|
|
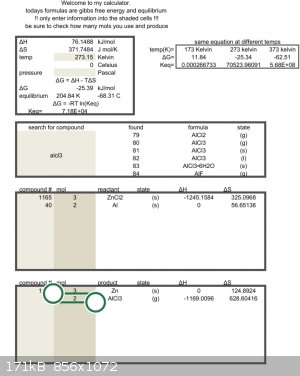
Got ya, so the calculator says silver chloride should work,
$$ 3AgCl + Al \longrightarrow 3Ag + AlCl_3$$
Then sublime the AlCl3 and dissolve excess AgCl in ammonia solution leaving fine silver powder.
Baaa I'm rambling again.
If you got a quarter and some HNO3, AgCl is easy to make and dry.
I'm on holiday and will try before Christmas.
[Edited on 21-11-2022 by Rainwater]
"You can't do that" - challenge accepted
|
|
|
sceptic
Harmless

Posts: 49
Registered: 7-6-2022
Location: Southern Africa
Member Is Offline
|
|
According to Wikipedia, sulfur trioxide has a boiling point of 45 Celsius, which is below that of water. If the white layer was sulfur trioxide,
wouldn't it have formed much farther from the hot end of the test tube, around the area where the water condensed?
|
|
|
Mateo_swe
National Hazard
   
Posts: 541
Registered: 24-8-2019
Location: Within EU
Member Is Offline
|
|
Have you seen Chemplayers video about preparation of anhydrous aluminium chloride from zink chloride and aluminium powder?
He doesnt get very high yields but the preparation is quite simple.
Here is a link to the video on bitchute:
Chemplayers preparation of anhydrous aluminium chloride
[Edited on 2022-11-21 by Mateo_swe]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Yep, that's exactly the method I reported here and on the reference (my now defunct site) CP quotes. For some reason he's moved the
video from Youtube to Bitchute.
It would be quite easy to improve on the measly yield of AlCl3.
[Edited on 21-11-2022 by blogfast25]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by sceptic  |
According to Wikipedia, sulfur trioxide has a boiling point of 45 Celsius, which is below that of water. If the white layer was sulfur trioxide,
wouldn't it have formed much farther from the hot end of the test tube, around the area where the water condensed? |
Possibly. That would be very easy to test.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4332
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
It might have been small droplets of sulphuric acid.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Good point, squire.
And here's my write up for the ZnCl2/Al method:
http://www.sciencemadness.org/talk/viewthread.php?tid=30150#...
Where have the years gone?
[Edited on 21-11-2022 by blogfast25]
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2786
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
If you can buy zinc chloride, why not buy aluminum chloride? I guess you could do the reaction for the interest of it, but usually AlCl3 is a stepping
stone to something else.
I guess you could make ZnCl2 from CuCl2 + Zn (less exothermic) and then use that for AlCl3, so the heat is released in two steps instead of all at
once.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Yes, of course.
|
|
|
| Pages:
1
2
3
4 |