Fery
International Hazard
    
Posts: 1053
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
benzalacetone = benzylideneacetone by aldol condensation
I followed orgsyn procedure at 8-times smaller scale.
http://orgsyn.org/demo.aspx?prep=cv1p0077
calculations
53,1 g benzaldehyde (0,5 mol) should be 51 ml
100 ml acetone (1,35 mol, big excess to reduce dibenzalacetone side reaction as well Cannizzaro side reaction of benzaldehyde by diluting benzaldehyde
into big volume of acetone)
50 ml water
12,5 ml 10% NaOH (1,25 g NaOH dissolved in water and topped to 12,5 ml)
Into 250 ml flat bottomed flask was added 53,1 g benzaldehyde free of benzoic acid (I weighed it directly into flask, did not measure its volume in
graduated cylinder to reduce its air oxidation), 100 ml of acetone, 50 ml of water. Thermometer inserted and the content was magnetically stirred.
Medicinal syringe was filled with 12,5 ml 10% NaOH and it was slowly dripped into reaction, covering the mouth of flask with a plastic foil to reduce
air entering and oxidation of benzaldehyde. T was kept 25-31 C by adjusting the rate of dripping, the reaction was slightly exothermic and the flask
was cooled by 15 C water bath. Dripping lasted 30 minutes. Cooling bath removed. Stirring continued for further 2 and half hour at room temperature so
the total reaction time was 3 hours.
Hydroxide was destroyed to prevent further multiple aldol condensations by dripping diluted HCl into reaction (5 ml 35% HCl diluted with water into
volume of 15 ml). Universal indicator paper showed that pH was initally strongly alkaline (that's good and a prove that benzaldehyde was not
contaminated with benzoic acid either it was not oxidized during reaction either Cannizzaro reaction did not create enough benzoic acid to poison the
catalyst NaOH). After neutralization with HCl the pH was slightly acidic.
Using separatory funnel the upper yellow layer was separated and bottom drained out and discarded. The yellow product was washed with 20 ml of water,
now it stayed as bottom layer and water as upper layer (very likely water colorless phase now as upper phase contained also with some acetone which
further reduced it density, maybe also some sideproducts of acetone aldol condensation like diacetonealcohol and mesityloxide?).
The product weighed now 95,1 g so it must contained also some acetone and condensation products of acetone.
It was dried with anhydrous CaCl2 (circa 2,0 g) overnight while magnetically stirring, then was let to settle. A lot of bottom colorless water phase
separated which was discarded, circa 10 ml (CaCl2 solution in water, maybe also some acetone and condensation products of acetone).
(Now I did not have time to process if further and it stayed in 100 ml stoppered flask for almost 1 month, maybe processing it immediately would give
better yield).
Vacuum distilled using water aspirator pump (vacuum circa -0,95 ... -0,97 atm) using lazy setup just to remove acetone and few drops of forerun which
condensated on the cold glass (circa 2-5 ml) to be sure that all acetone as well water was removed, also some mesityl oxide (b.p. 130 C at 1 atm) and
diacetone alcohol (b.p. 166 C at 1 atm) very likely passed into receiving flask.
Then lazy setup removed and attached regular water condenser, themometer, Anshutz-Thiele adapter and connected to 2-stage oil rotary vane vacuum pump
(my mechanical vacuum gauge is unable to measure such low pressures and it just shows 0 atm). Initially cold water circulated through condenser and
product solidified in the adapter as well condenser. Warm water 50 C was passed through condenser to melt the product so it slid into the receiving
adapter. Collected distillate at 86-95 C. Receiving adapter heated to melt the product, product then poured into a dish with a bed from aluminium
foil, let to solidify and crushed to smaller pieces.
yield 44,3 g = 0,30 mol = 60%
useful info gathered using internet:
Molar mass 146.19 g/mol
Appearance pale yellow solid
Density 1.008 g/cm3
Melting point 39 to 42 °C (102 to 108 °F; 312 to 315 K)
Boiling point 260 to 262 °C (500 to 504 °F; 533 to 535 K)
Solubility in water 1.3 g/L
The product could be oxidized with NaOCl into cinnamic acid (haloform reaction). It can also be hydrogenated into 4-phenyl-2-butanone with floral and balsamic scent.
Using Anshutz-Thiele adapter was unnecessary, I was just prepared to fractionate the product. But former lazy water aspirator pump predistillation
removed all foreruns and then regular vacuum distillation distilled immediately the desired product. Using warm water circulation was necessary
otherwise the product solidified in condenser. If possible try not to use vacuum take off adapter with narrow tube, the tube could clog. Maybe using
2-neck receiving flask connected directly to the condenser and vacuum connected to the second neck would be safe setup (similarly as seen on my lazy
vacuum predistillation setup). Be prepared that distillation setup could get obturated and try to avoid that as much as possible.
beginning of the reaction, in the flask there is benzaldehyde + acetone + water + stir bar

dripping solution of NaOH into reaction, as seen the reaction was slightly exothermic, the mouth of the flask was covered by a plastic foil to reduce
air entering and oxidation of benzaldehyde thus destroying catalyst (by reaction into sodium benzoate), after the addition the reaction was further
stirred
 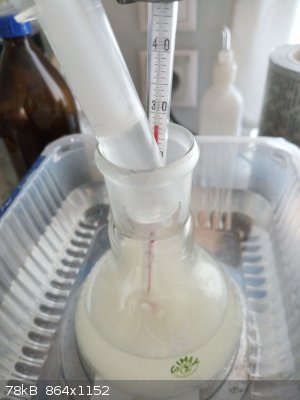  
the reaction stopped by neutralization of NaOH by diluted HCl, universal indicator paper showed initially strongly alkaline pH (left blue paper) and
slightly acidic after the neutralization (right reddish paper)

after the neutralization the mixture was allowed to settle
  
using separatory funnel the upper yellow organic phase was separated and bottom aqueous phase discarded (its density was higher very likely due to
NaCl)
then the yellow organic phase was washed with water but now the water phase was as upper layer and the organic phase was bottom layer... densities of
the product and water seems to be quite close together

drying with CaCl2, still surprisingly a lot of water phase (colorless) on the bottom due to higher density thanks to CaCl2, using only as little of
CaCl2 as dissolves is enough, no need to waste organic layer adhering to crystals of CaCl2 hydrate

lazy predistillation setup to remove acetone, water, diacetonealcohol, mesityloxide, acetone does not condense and passes directly into water
aspirator that's OK, a little of colorless sideproduct collected, circa 5 ml (could be diacetonealcohol or mesityloxide)
  
vacuum distillation using stronger vacuum (2 stage oil rotary vane vacuum pump) and regular setup including thermometer and condenser...
Anschutz-Thiele adapter later revealed to be unnecessary, predistillation removed all forerun, instead of the adapter also 2 neck receiving flask
could be directly connected to the condenser (similarly as used in predistillation)

first drops of the product in condenser, a moment with very nice feeling...
 
but no one pleasure lasts forever and no drop collected in the receiving flask connected to the Anschutz-Thiele adapter... further investigation
showed that the product solidified soon... first in the adapter and later also in the condenser... there is an initial period when first crystal
appears and then the crystallization goes faster and from the coldest part upwards against flow
     
|
|
|
Fery
International Hazard
    
Posts: 1053
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
too much photos so the post had to be split into 2 posts due to max number of photos limitation
cold circulation water was changed into 50 C warm water to melt the product to allow it to flow
  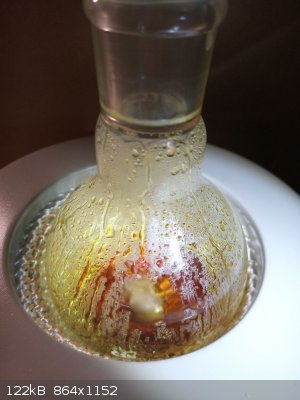   
finally the receiver was warmed to melt the product completely

and liquid product was drained into a dish with aluminium foil bed in which it was let to solidify
  
|
|
|
Bedlasky
International Hazard
    
Posts: 1251
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
Nice work, Fery!
Does benzalacetone smell? Dibenzalacetone have weak sweet smell, so I wonder if benzalacetone have similar smell. Do you have further plans with this
compound?
[Edited on 15-11-2022 by Bedlasky]
|
|
|
Fery
International Hazard
    
Posts: 1053
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
Hi Bedlasky, its scent is nice, some kind of sweet, but hard to describe.
Dibenzalacetone should be in the remainder in the distillation flask after vacuum distillation, but it was quite dark and contaminated with some aldol
polycondensation compounds so I discarded it.
I would like to try the haloform reaction into cinnamic acid and hydrogenation of double bond into that floral scenting compound (requires Ranney
nickel or better Pd on C or even better Rh on Al2O3) similarly as rheosmin: https://asset-pdf.scinapse.io/prod/1976016712/1976016712.pdf
|
|
|
|