teodor
National Hazard
   
Posts: 922
Registered: 28-6-2019
Location: Netherlands
Member Is Offline
|
|
(iso-)amyl salicylate - the most intriguing ester
From this article:
http://agri.ckcest.cn/ass/43f5549a-582c-412c-a9e0-c933bd52eb...
"The first example in fine fragrance was probably isoamyl salicylate,
discovered early on by Darzens and used in 1898 by Jacques Rouché
(both at the Piver company) in the perfume ‘Trefle incarnat.’ Patricia
de Nicolaï in her article A Smelling Trip into the Past: The Influence
of Synthetic Materials on the History of Perfumery notes that ‘it was
a real revolution in the Fougère family.’
The published procedures of preparation are:
1. iso-amyl salicylate - C. Drion, Compt. rend., 39, 122 (1854) - https://www.biodiversitylibrary.org/item/21179#page/132/mode...
2. n-amyl, 2-amyl, tert-amyl salicylates - Andrew F. Freeman and H. L. Haller, "The Preparation of Amyl Salicylates", https://doi.org/10.1021/ja01276a502
I have prepared n-amyl and iso-amyl salicylates. I didn't find those articles at that moment so I used my own procedures:
1. For n-amyl salicylate
35.4g salicylic acid (S3 chemicals, 99.5 - 100.5%, Ph. Eur., USP)
22ml n-amyl alcohol (S3 chemicals, 99%)
50ml purified toluene
0.2g p-toluenesulfonic acid dihydrate
Dean-Stark method (with the toluene as a water carrier)
My trap has a fixed volume of 20.3 ml.
Salicylic acid is completely dissolved during warming.
It looks like either p-toluenesulfonic acid is not a good catalyst or the temperature is low, the water collection in the Dean-Stark trap is very slow
and the yield is poor. The progress of water collection is (the volume @ the time)
0 @ 9:23 pm (start)
0.5ml @ 0:16 am
1.3ml @ 4:43 am
2ml @ 12:14 am
at this time 6ml of H2O was added to push toluene (mixed with alcohol, so it keeps some quantity of the alcohol out of synthesis) from the Dean-Stark
The process was stopped at 4:44 pm
200ml of H2O was added to the flask. With cooling the salicylic acid excess was crystallized, so 12g of NaHCO3 was added and stirred. The sodium salt
was completely dissolved (it is important to remove the acid because it is prone to thermal decomposition on later stages forming quite smelly
products).
The bottom layer was separated and washed twice with 100ml of water.
The toluene from dean-stark was saturated with NaCl and the top layer was added to the product.
30ml of toluene was distilled off using a widmer column. During this process the temperature in the source flask reaches 200C.
The water was added to the product in the source flask (attention: cool it first, otherwise you will get the "champagne effect", I've got it so I lost
some product at this stage).
So, 150ml of water was added, separated, and washed 1 time with H2O, NaHCO3 (but it wasn't required), and 3 times with water.
I didn't distill the product because it was too little.
2. iso-amyl salicylate
iso-amyl alcohol (chemship1978) was dried for 1 day on K2CO3 and distilled through a widmer column, the middle fraction was collected. chemship1978
has very good quality alcohol, most of it passes 131-131.5C. Probably it has a synthetic origin. My previous experience with iso-amyl alcohol from
Restauro Online was quite different, 5 fractions were collected and 3 middle fractions were in the 130 - 131C range every of which had a quite
different smell. I think Restauro Online alcohol is not synthetic one but the result of fermentation.
17.2g salicylic acid (S3, as before)
36ml 131-131.5C iso-amyl alcohol
5ml conc. H2SO4 (attention: slowly poor (from a beaker, not from a pipette!) in the alcohol cooled in ice with a good stirring; failing to do so
creates some irregularities as the result of the alcohol caramelization which change the way of synthesis because they accumulate the heat)
3hr of reflux with the temperature control - it should never go over 134C (otherwise the preparation will be spoiled - it creates some awful-smelling
byproduct)! Also, the reflux should be stopped either when the color of the mixture becomes yellow-brown or the smell of unsaturated hydrocarbon on
the top of the cooler becomes too apparent.
400ml of filtered water was added to the result. It often forms emulsion which requires the usage of some solvent to break. I used CCl4 but diethyl
ether could be used also.
The result of separation was washed 1 time with an equal volume of water, 1 time with saturated NaHCO3 (it showed no bubbling, probably the pH of
filtered water was enough to neutralize the rest of the acids), 2 times with water, dried 1 night over MgSO4. CCl4 was distilled over through a Widmer
column and the rest was subjected to vacuum distillation. iso-amyl sallicilates passes 147C (probably 149C, the thermometer is not calibrated at this
range yet) @ 24mmHg. The yield is ~20ml.
But I wrote "the most intriguing ester(s)".
My samples of both esters (n-amyl and iso-amyl) have almost the same smell in concentrated form. It is smell of some burned plastic. I was thinking it
could be the result of over-heating during fractionation (but I never had a problem heating methyl salicylate up to its boiling point at atm pressure,
so I doubt it). But smell changes dramatically on dilution. The most intriguing that it is much dependent on the media. The fresh air and mineral oil
(10 drops per ~7ml of oil) have one characteristic, but most interesting are experiments with alcohols. There is no big difference between 3000pm and
3ppm in ethanol but it is completely different with 3000-12000ppm in iso-amyl alcohol. The smell of diluted esters is good and complex. For me, it has
nothing in common with methyl or ethyl salicylate.
I am a bit puzzled. Reading data from internet sites about the esters I never found this information about bad smell until dilution and so big
dependence on the carrier.
Probably I will do a few more experiments with salicylates to understand if the bad smell in high concentration is the result of improper procedure,
personal smell perception or the ester should be always diluted.
I think H2SO4 should be used as a catalyst together with a low-temperature dean-stark and all fractionation should be performed under vacuum to
exclude over-heating and oxidation of the ester.
I am still not so brave as to read the original preparation from 1854 in French but I will try.
Update: I've printed the french article and used Google Translate from my android phone. Indeed, the author was failed to make iso-amyl salicylate by
"usual procedure", so he made salicyl chloride and acts with it on amyl alcohol. So, my "burning plastics" probably are not pure amyl salicylate
esters! Probably as the ester has a smell detectable in sub-ppm amounts and the "burned" component is not the dilution works to reveal an ester smell
in these cases.
(Also M. Ch. Drion pointed out that the most abundant byproduct was "l'hydrate de phenyle" - I have no idea what is it but I suspect it is not a fine
fragrance).

[Edited on 29-6-2022 by teodor]
|
|
|
Fery
International Hazard
    
Posts: 1026
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
Hi teodor, interesting experiments!
My experience with Dean-Stark method (but different esters) is that the reaction was complete in circa 1 hour, I used isohexanes to drive out water.
Toluene is even more powerful and xylene better than toluene. I used isohexanes because b.p. of mine esters were much lower than of your esters.
Sulfuric acid quantity - I use 1 ml per 1 mol of ester. Using too much H2SO4 caused carbonization and at the end of the reaction the acid was
insoluble and separated at the bottom like here: https://www.sciencemadness.org/whisper/viewthread.php?tid=15...
|
|
|
Texium
Administrator
       
Posts: 4618
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Thanks for another good write up, teodor! Regarding “l'hydrate de phenyle,” that sounds like an alternative name for phenol, which would make
sense as a byproduct of overheated salicylate, and perhaps partly explain the burnt plastic odor that you observed.
|
|
|
teodor
National Hazard
   
Posts: 922
Registered: 28-6-2019
Location: Netherlands
Member Is Offline
|
|
@Fery
Thanks, my experience with Dean-Stark is also quite good for making various esters. But in this case, the process is really slow and far from
completion and I think the reason is p-TsOH doesn't work as a catalyst here.
I personally would not use ordinary xylene isomers mix to make esters. I spent a lot of hours (with this distillation rig, by the way 

studying my xylene composition. I've separated several fractions and the last one has a smell of cow dung. Typical toluene also has some sulfur
compounds but they could be removed quite well with a help of sulfuric acid. But for xylene, it doesn't work because of the easiness of sulfonation of
xylene itself. For the same reason, I wouldn't use either xylene or toluene with H2SO4 as a catalyst. I think benzene or hexane would be much better
because it is much more inert to sulfonation.
@Texium
I feel like I don't have enough patience & knowledge of the language for making good write-ups, so thanks for reading my rough sketches.
I am familiar with salicylic acid decomposition to phenol, but the smell I've got is not a smell of phenol. This burning smell never appears during
methyl or ethyl salicylate synthesis (while the decomposition of the acid to phenol happens, that's why I have that trick of neutralization of the
acid with NaHCO3 before any distillation). Probably I will try to make butyl salicylate which is something in the middle of the range to understand
what can happen with high molecular alcohols here.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4355
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
I think I made iso-amyl benzoate once. I didn't find the smell very note-worthy.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Fery
International Hazard
    
Posts: 1026
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
Hi Teodor, if you use H2SO4 as a catalyst and toluene as an entrainer then some amount of PTSA is present in the reaction. Perhaps also when you use
only PTSA some amount undergoes desulfonation into H2SO4 + toluene, but maybe not (it is catalyzed by H2SO4 and high temperature and when using pure
PTSA the H2SO4 is not present at least at the beginning). Yes hexane is completely inert. I use hydrogenated medicinal petrolether which is a mixture
of isohexanes. It is less powerful in removing water (les water % in vapor phase) and harder to condense (b.p. of the mixture isohexanes+h2o could be
maybe 50 C or maybe even less, I did not calculate either measure that).
|
|
|
teodor
National Hazard
   
Posts: 922
Registered: 28-6-2019
Location: Netherlands
Member Is Offline
|
|
I noticed that it is an acid component which is responsible for ester smell. The alcohol constituent plays the second and quite predictable role, at
least for aliphatic alcohols.
The smell of a methyl ester is almost the "pure acid" smell. Ethyl is very close.
The middle of "the ester" line where a really new smell appears are butyl and amyl. Amyl is always more sweet and butyl is more bitter but they are
always close to each other and are most characteristic alcohols to reveal the smell of the whole line.
Secondary alcohols reveals quite different smells from primary ones.
So, I think there could be nothing in common between amyl salicylate and benzoate except that "sweetness" which is the smell characteristic of amyl
alcohol.
On the other hand, I don't notice anything in common between methyl and amyl salicylate. I think because the smell in the first members of the line -
methyl and ethyl - are masked by salicylic acid smell which is kind of "aromatic" - close to naphtalene and toluene, and this doesn't allow to reveal
more complex "ester" smell which I think should start from butyl. Also I think that many samples of methyl/ethyl salicylate esters could be
contaminated with salicylic acid decomposition products.
[Edited on 30-6-2022 by teodor]
[Edited on 30-6-2022 by teodor]
|
|
|
Fery
International Hazard
    
Posts: 1026
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
Hi teodor, you are right with alcohols. I wanted to prepare cocoa esters (butyl and n-amyl cinnamates) but did not yet have time for that.
http://www.thegoodscentscompany.com/data/rw1013181.html
http://www.thegoodscentscompany.com/data/rw1048861.html
I have commercial methyl cinnamate and it should have strawberry scent, but unfortunately for uknown reason I did not scent almost anything (it is
solid, in form of crystals and strange that butyl as well amyl should be liquid).
http://www.thegoodscentscompany.com/data/rw1417571.html
|
|
|
DraconicAcid
International Hazard
    
Posts: 4355
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Sprinkle a bit of methyl cinnamate into a beaker of warm water if you want to smell the strawberry.
Methyl esters often have higher melting points than longer-chain esters, due to the way the molecules pack.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Fery
International Hazard
    
Posts: 1026
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
My best experience is by using about 1 ml of H2SO4 per 1 mol of ester and dean stark with isohexanes (medicinal petrolether so the hydrocarbons are
halogenated and no unsaturated hydrocarbons present). H2SO4 is only a catalyst. Too much H2SO4 and too high temperature would make also the
corresponding dialcoholether from the alcohol. That's why I use isohexanes and dean stark and only a little of H2SO4, because then the reaction
proceeds at temperature approx somewhat around 60 C or only slightly more (b.p. of hexanes cca 60 C, bp. hexane+water maybe cca 50 C, but the temp in
the reaction flask would be increased due to dilution in a lot of higher boiling point compounds).
Salicylic acid has also -OH group and there could be this intermolecular esterification side reaction HO-C6H4-COOH + HO-C6H4COOH ->
HO-C6H4CO-O-C6H4COOH + H2O
Making methyl salicylate is esterification in 10-fold excess of methanol to reduce intermolecular esterification of salicylic acid.
But making ester with expensive alcohols cannot be done that ineffective way in 10-fold excess of expensive alcohol.
Maybe the way is making a bromide from the expensive alcohol and then reacting the halocarbon with Na salicylate?
Protecting OH group of salicylic acid with acetic anhydride by making acetylsalicylic acid first is not good idea because when removing this
protecting group that would also hydrolyze the ester we would like to synthesize.
|
|
|
teodor
National Hazard
   
Posts: 922
Registered: 28-6-2019
Location: Netherlands
Member Is Offline
|
|
Thanks for your insights, Fery.
Yes, probably only few people know (Fery) that I continue to do experiments with salicylic acid.
Unfortunately, not so much to share yet.
Yesterday I tried to make salicyloyl chloride by SOCl2 method but it went wrong. After initial (several hours) dissolution of salicylic acid the
result begin to solidify with continous bubbling and instead of getting liquid melting 18C I've got a white powder. There is still some chloride (I
was able to detect the smell of ester after addition of the alcohol) but it's evident the SOCl2 method is unable to produce the pure product. I think
the reason is that OH group interfere here. My (stupid) guess is that after forming "unstable nucleofillic compound" with SOCl+ it doesn't go as
expected, so it doesn't form leaving group but makes a ring closure over S atom... of course it is just stupid guess. So, I'd like to protect OH group
somehow to see how it can interfere with the reaction path. From what I read it should go smooth with acetilsalicylic acid.
Also, as McMaster & Ahmann said (J. Am. Chem. Soc, 1928 (50) p. 148) "we were able to obtain by using the free acid only a very small yield of the
acid chloride of salicylic acid but a 36% yield was obtained by using the sodium salt of the acid .... No yield of the acid chloride could be obtained
with m-hydroxybenzoic acid nor with o-and m-cresotinic acid...".
So, I am thinking now why the reaction with SOCl2 is so dependent on -OH group position and also why it goes better with Na salt of the salicylic
acid. There are no answers so far.
There is interesting method of acetylsalicylic acid chlorination using urea as a catalist. And I have no idea how urea works here ...
https://prepchem.com/stage-1-preparation-of-acetyl-salicyloy...
|
|
|
Pumukli
National Hazard
   
Posts: 708
Registered: 2-3-2014
Location: EU
Member Is Offline
Mood: No Mood
|
|
Came across this thing:
"Synthesis of n-Amyl Salicylate Catalyzed by Sodium Hydrogen Sulfate
Ma Rong-xuan
Published 2009
Chemistry
n-Amyl salicylate was synthesized from salicylic acid and n-pentanol using sodium hydrogen sulfate as catalyst.Factors influencing the ether
yield,such as molar ratio of alcohol to acid,catalyst dosage,reaction time,water carrier dosage were discussed.Experimental results showed that sodium
hydrogen sulfate was an excellent catalyst.The optimum synthetic conditions were as follows: molar ratio of salicylic acid to n-pentanol was
1∶1.5,catalyst dosage was 4.7% of mass of the reactants,using 10 mL of cyclohexane as water carrier,reaction temperature was 120~140℃ and reaction
time was 4.5 h.Under above conditions,the yield of n-amyl salicylate achieved 93.3%. "
Dunno... May be useful one day for someone. 
A similar one from some obscure source:
"Synthesis of Amyl Ferulate Catalyzed by Sodium Bisulfate Supported by Silica
October 2014Advanced Materials Research 1033-1034:30-33
DOI:10.4028/www.scientific.net/AMR.1033-1034.30
Authors: Xiang Wen Kong, Huan Wang, Han Wang, Li Li Ren
To read the full-text of this research, you can request a copy directly from the authors.
Abstract
Amyl ferulate was synthesized by direct esterification with using sodium bisulfate supported by silica as a catalyst, ferulic acid and n-pentanol as
raw materials. The influences of some factors on the synthetic process were studied. The optimal reaction conditions based upon 0.2 mol of ferulic
acid were chosen that the molar ratio of n-pentanol and ferulic acid was 2: 1, the mass ratio of catalyst to reactants was 6%, refluxing reaction time
was 3 hours. The yield of the product reached 97% under the above conditions. The structure of the product was characterized by IR, 1H NMR and MS
spectrum. The catalyst could be recycled and used for many times."
[Edited on 27-7-2023 by Pumukli]
|
|
|
Fery
International Hazard
    
Posts: 1026
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
To have perfume grade product maybe the way is transesterification of methyl salicylate with longer chain alcohol, methanol would just distill off the
reaction. Also using only very little of catalyst (H2SO4) or maybe better NaHSO4 which is weaker acid as the first hydrogen of H2SO4 is neutralized
and for the second one pKa2 = 1,92. That seems enough acidic as formic acid is able to esterify itself without additional catalyst (pKa 3,75 which is
much more weaker than the second proton of H2SO4).
|
|
|
teodor
National Hazard
   
Posts: 922
Registered: 28-6-2019
Location: Netherlands
Member Is Offline
|
|
I think I solved the mistery of salicyloyl chloride solidification. In the presence of SOCl2 the reaction is not stopped on forming salicyloyl
chloride but goes further when two molecules of the chloride reacts to form the depside
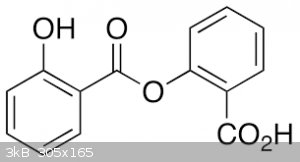
(diplosal or salicyloylsalicylic acid) m.p. 148C (Rodd's chemistry of carbon compounds, vol. IIId, also it is exactly what Fery said in regard to side
esterification reaction). So I have to check the melting point after this side-product purification. Also, it's now clear that the synthesis of
salicyloyl chloride should be stopped just after all salicylic acid is dissolved.
Hm, this form looks "chelating"
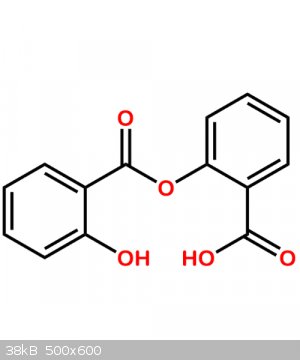
[Edited on 27-7-2023 by teodor]
|
|
|
Fery
International Hazard
    
Posts: 1026
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
I wonder whether the remaining OH and COOH of the salicyloylsalicylic acid cannot esterify to create 3 ring molecule or if 3 molecules of salicylic
acid cannot trimerize in a chain...
|
|
|
teodor
National Hazard
   
Posts: 922
Registered: 28-6-2019
Location: Netherlands
Member Is Offline
|
|
As far as I know such reactions could be stimulated by some metals which would form a center around which the cycle forms. The metal shold coordinate
to 3 oxigens. And it should be very small atom to fit there.
The next open problem is to find suitable solvent. The dimer is very unsoluble but was studied once as possible aspirin replacement.
And if you will be able to build cyclic trimer from salicylic acid probably you will be able to build a nice motif from an acid with 2 OH groups.
[Edited on 28-7-2023 by teodor]
|
|
|
teodor
National Hazard
   
Posts: 922
Registered: 28-6-2019
Location: Netherlands
Member Is Offline
|
|
@Fery
 
[Edited on 28-7-2023 by teodor]

[Edited on 28-7-2023 by teodor]
[Edited on 28-7-2023 by teodor]

|
|
|
teodor
National Hazard
   
Posts: 922
Registered: 28-6-2019
Location: Netherlands
Member Is Offline
|
|
I found the ultimate guide for salicyloyl chloride synthesis. In the russian patent https://patents.google.com/patent/RU2601309C1/ru.
The conclusion: SOCl2 method gives up to 3% of sulfur compounds in the product. It's not suitable for fine fragrance 
The problem with KHSO4 I think the same. In my opinion, sulfur should be avoided. At least for making a reference sample.
|
|
|
teodor
National Hazard
   
Posts: 922
Registered: 28-6-2019
Location: Netherlands
Member Is Offline
|
|
Probably it is US2899458 that disclosed the most accurate method of salicyloyl
chloride preparation by SOCl2.
I think it is close to the original (probably not published) procedure
invented by Georges Darzens (the author of the first perfume-grade
isoamyl salicylate preparation) who also discovered a role of tertiary
amine bases in preparation chlorides from alcohols.
When I did the study of the topic by the excelent book of Patai "The
chemistry of acyl halides" (the part of the serie "The chemistry of
functional groups") in the section of the preparation chlorides from
aromatic acids with unprotected -OH groups there was mentioned that it is
tertiary amine base (pyridine) which makes the reaction go in the proper
direction of forming the acid chloride.
The method disclosed in that patent of 1955 (57 years after inventing
of Darzens method) is excelent in several points:
1. It uses a slight excess of salicylic acid over SOCl2 which is
unusual but eliminates the need of distilling-off the excess of the
reagent which according to my observation can shift the reaction into
the side of forming depside.
2. It uses 30C as the reaction temperature to minimise the side reactions.
3. It uses light solvent (pentane-hexane mixture) to perform all operations (uncluding distilling-off the solvent) under 60C.
I didn't have hexane, so I used n-heptane having intention to use the chloride in the same heptane solution for esters preparations.
1 week before I started SOCl2 purification by classical method - distilling from quinoline to eliminate acids and after that from linseed oil to
remove yellow-colored sulfur compounds.
 
After this treatment I've got colorless clear liquid not much fuming on air (low dissolved HCl cotents after quinoline treatment).

Salicylic acid USP grade (S3 Chemicals) was kept several weeks in a dessicator over conc. H2SO4 and had a form of dry white powder.
In 100 ml flask I poured 50ml of n-heptane 99%+ (laboratoriumdiscounter.nl, "n-Heptaan").
Then I've put a magnetic stir bar followed by 6.362g of "Salicylsäure".
Then I've dropped ~0.035ml of pyridine and started stirring.
To this mixture 3.45ml of purified SOCl2 was added.

The water bath temperature was kept in the range 27 - 42C by changing
water. In several hours stirring at 1500rpm mostly all salicylic acid
disapearred. The liquid was filtered and put in freezer <-12C. In 1-2
hours many small needle-like crystalls where formed but it was still
small amount of them, either they are soluble or I should wait a bit
longer.

@Fery: I'll relay on you in experimenting with alcohol part 
"Vector synthesis" of 9 salicylic esters:

[Edited on 30-7-2023 by teodor]
|
|
|
teodor
National Hazard
   
Posts: 922
Registered: 28-6-2019
Location: Netherlands
Member Is Offline
|
|
So, my conclusion:
Salicyloyl chloride method gives the same smell of iso-amyl salicylate as H2SO4/azeotropic method which is quite bad in some concentration range. To
reveale the true delicious smell of this ester it should be diluted to 3ppm in neutral solvent like paraffine oil (the synthetic path doesn't truely
matter on such dilution).
Some other things can change the smell perception:
1. Dilution the ester with water change it to the "bad smelling" side (even at low concentration).
2. Dilution the ester with HCl (SO2?) change it to the "good smelling" side (even at high concentration).
Another interesting smell is 2-butyl salicylate. Basically, it is closely related but lacks the "harness" of iso-amyl varietty.
Both n-amyl and n-butyl are too "plain/sharp" and I doubt they are worth to try. As well I didn't find 3-butyl interesting.
[Edited on 31-7-2023 by teodor]
[Edited on 31-7-2023 by teodor]
[Edited on 31-7-2023 by teodor]
|
|
|