DocX
Hazard to Others
  
Posts: 179
Registered: 22-10-2015
Member Is Offline
Mood: No Mood
|
|
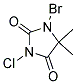
Making bromine from Bromchlor-5,5-dimethylimidazolidin-2,4-dion
So Sodium bromide is not used anymore for pool chemistry where I'm from. Instead, the bromine content comes in the shape of
Bromchlor-5,5-dimethylimidazolidin-2,4-dion
Does anyone know if and how this can be turned into pure bromine in a home lab?
And yes, I know about the dangers of bromine. I have studied the classic conversion of sodium bromide and TCCA with HCl to bromine in detail. But this
is a different entity. Looking at the schematic, the bromine is attached to the imidazolidine with a nitrogen, what does it take to separate it? And
won't I get a lot of chlorine gas too, since the chlorine has the same bond?
[Edited on 20222222/6/26 by DocX]
|
|
|
mackolol
Hazard to Others
  
Posts: 459
Registered: 26-10-2017
Member Is Offline
Mood: Funky
|
|
https://www.youtube.com/watch?v=zZKh2RvfWBY&ab_channel=E...
https://www.youtube.com/watch?v=_bfHr7IAd6Q&ab_channel=A...
https://www.bitchute.com/video/RNssjAbGpYXL/
Literally typed that on youtube, don't be discouraged if something looks a bit confusing, sometimes simplest solutions are the key.
[Edited on 26-6-2022 by mackolol]
|
|
|
DocX
Hazard to Others
  
Posts: 179
Registered: 22-10-2015
Member Is Offline
Mood: No Mood
|
|
Funny, I typed in Bromchlor-5,5-dimethylimidazolidin-2,4-dion on YouTube also and got results like "Finasteride microdosing"? Strange.
And when adding "making bromine from" I got a lot of videos showing how to make it from NaBr, which I already know by heart.
I LITERALLY got none of the hits you have there. I guess my problem was I was unaware of the abbreviation.
But thank you so much! Those come in very handy!
Also, I should say I did find parts of the answer smeared out across like four pages of the bromine synth sticky after posting this thread. But like
so many threads, it's almost inaccessible due to lost pictures and too much arguments for anything to be apparent. This thread is now however, thanks
to you, a pinnacle of clarity!
Except ... it does seem to produce BrCl-contaminated bromine, doesn't it? I read in the messy bromine synth sticky that using KBr could prevent this.
But really, if I had KBr, why on earth would I do this reaction in the first place?
[Edited on 20222222/6/26 by DocX]
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Tdep has done this on Extractions and Ire.
A full procedure and write up was done by len1 and published in his excellent book "small scale synthesis". I can probably post the relevant chapter
if you can't find it.
He does deal with the chlorine problem from both a theoretical and analytical standpoint. I forget the details except to say the product is quite
pure.
|
|
|
Texium
Administrator
       
Posts: 4580
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Quote: Originally posted by DocX  | Funny, I typed in Bromchlor-5,5-dimethylimidazolidin-2,4-dion on YouTube also and got results like "Finasteride microdosing"? Strange.
And when adding "making bromine from" I got a lot of videos showing how to make it from NaBr, which I already know by heart. |
That’s the German name you’re using. In English it is usually called 1-bromo-3-chloro-5,5-dimethylhydantoin, which is where we
get the BCDMH abbreviation.
|
|
|
Fantasma4500
International Hazard
    
Posts: 1681
Registered: 12-12-2012
Location: Dysrope (aka europe)
Member Is Offline
Mood: dangerously practical
|
|
i have about.. 5kg of this stuff, ascorbic acid also works it seems
but what the hell do i do with the end product? im a bit of a collector but prefer collecting universally useful materials, liquid bromine is a bit on
the risky side to have.
HBr? bromoform? it has potential for brominating solvents which can then maybe be turned into amines and then oxidized into alkyl energetics. DCM is
supposedly a nice and safe way to store bromine but DCM isnt very easy to get anymore
metabisulfite is mentioned, this would imply SO2, along with already bromine and chlorine- could an ammonia trap be used to catch vapors and liquid,
resulting in ammonium chloride-bromide-sulfite? would bleach be a concern for Cl2 + NH4OH? or what other reducing agents may we use for this. maybe
reacting this whole thing with NaOH in hopes of achieving a naïvely easy to seperate organic mess from the inorganic sodium chloride/bromide?
maybe NaOH + glucose electroreduction, or would that maybe end up also reacting once again with the formed NaBr or can this just be countered by
adding in excess hydroxide? i would hate to work with this organic mess and turn it into inorganic asap
|
|
|
DocX
Hazard to Others
  
Posts: 179
Registered: 22-10-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by j_sum1  | Tdep has done this on Extractions and Ire.
A full procedure and write up was done by len1 and published in his excellent book "small scale synthesis". I can probably post the relevant chapter
if you can't find it.
He does deal with the chlorine problem from both a theoretical and analytical standpoint. I forget the details except to say the product is quite
pure. |
Ok, great. I have the book, forgot to check it. Thank you!
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2787
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
The simplest way to "store bromine" is by forming the salts KBr and KBrO3, which are both shelf-stable, and which, combined in a 5:1 KBr:KBrO3 molar
ratio with a little acid, produce bromine. In particular, if all of the bromine is converted to KBrO3, simply heating this to ~350 C returns it to the
bromide. KBrO3 precipitates from cold aqueous solution (sol. ~3% w/w at 0 C)
Ampoules are more convenient if you have the equipment.
|
|
|
Mateo_swe
National Hazard
   
Posts: 541
Registered: 24-8-2019
Location: Within EU
Member Is Offline
|
|
Just turn ordinary test tubes into ampoules with a propene torch.
Fill them with the bromine and use the torch to seal them shut in the top.
As long as the ampoule doesn't break the bromine stores like forever without any leakage.
Watch youtube videos on making the ampoules, its not hard.
|
|
|