Rainwater
National Hazard
   
Posts: 919
Registered: 22-12-2021
Member Is Offline
Mood: indisposition to activity
|
|
Formal charge
Started reading organic chemistry 8th edition (paula yurkanis bruice)
Page 15. Problem 16a.
The answers isnt in the back of the book.
Google doesnt know what this molecule is and i want to check my work.
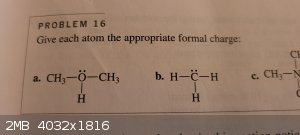
Here oxygen has 3 bonds and 2 lone pairs. 8 electrons total.
Formal charge = # of valence electrons - (lone pairs + bonds)
6 - (2 + 3) = 1 so the formal charge is "+1"
Whats throwing me for a loop is what i was taught is oxygen only wants to form 1 or 2 bonds. Here it has 3.
"You can't do that" - challenge accepted
|
|
|
BromicAcid
International Hazard
    
Posts: 3245
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Yup, formal charge of +1.
Oxygen usually only likes to form 2 bonds, but the material in A is a common intermediate in ether cleavage. In this case, your ether CH3-O-CH3
(dimethyl ether) would be treated with a strong acid forming this intermediate, protonating the oxygen. Then the other half of the acid, providing it
is a strong enough nucleophile like iodine (from hydroiodic acid) would come in and knock off an alcohol (a good leaving group) and leave behind the
methyl iodide. So oxygen doesn't like to have these three bonds but that is what makes it a good intermediate.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4332
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
An oxygen with three bonds and one lone pair has a formal charge of +1, just like in the hydronium ion. And since it only wants two bonds, it's not
happy.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|