vano
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
Chloramine-T, making iodine monochloride
Hi, i bought Chloramine-T, i read "It converts iodide to iodine monochloride (ICl)" in wikipedia. i'm just interested if i can make pure iodine monochloride
with this reaction. I need practical advice. If anyone has another interesting idea tell me.
|
|
|
vano
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
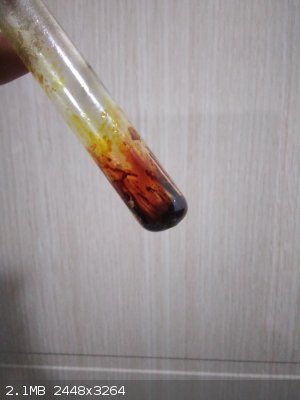
It works. I just heated up mixture of Chloramine-T and iodine. I will try larger volumes.
|
|
|
sauveurdumonde
Harmless

Posts: 33
Registered: 13-7-2021
Location: Canada
Member Is Offline
|
|
That's very cool, now, what do you plan to use the ICl for?
This isn't exactly a useful synthesis, but you could try reacting it with unsaturated fats, and the resulting halogenated fat with NaOH, making
chloro-iodo "soaps". Then compare the soap's properties based on whether or not it's halogenated or something.
Just a fun idea, even if it's a waste of precious iodine
"There is a shadow of a nation behind you, the hope of a people... yet it may not matter. The Divide still stands against us."
|
|
|
vano
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
interesting idea thanks. to tell you truth i don't have idea about the interhalogen, in this particular reaction, i was more concentrated on
properties of chloramine - T, than product itself.
|
|
|
Bedlasky
International Hazard
    
Posts: 1240
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
Quote: Originally posted by sauveurdumonde  | That's very cool, now, what do you plan to use the ICl for?
This isn't exactly a useful synthesis, but you could try reacting it with unsaturated fats, and the resulting halogenated fat with NaOH, making
chloro-iodo "soaps". Then compare the soap's properties based on whether or not it's halogenated or something.
Just a fun idea, even if it's a waste of precious iodine |
Actually, this reaction is very useful in analysis of food. Iodine monochloride and iodine monobromide are used to determine iodine number of oils.
Excess of reagent is then determined by iodometry.
|
|
|