| Pages:
1
2 |
danieldavies
Harmless

Posts: 19
Registered: 31-5-2019
Member Is Offline
|
|
Sodium chloride and sodium nitrate seperation.
How would I go about separating a solution of sodium chloride and sodium nitrate? I want to reuse the sodium nitrate for making nitric acid.
I dissolved silver in nitric acid to form silver nitrate. I then added sodium chloride to precipitate silver chloride. I decanted the solution from
the silver chloride. This solution is sodium nitrate contaminated with sodium chloride?
I am looking to get a pretty pure product back?
|
|
|
j_sum1
Administrator
       
Posts: 6334
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Why bother? Use it as is.
|
|
|
danieldavies
Harmless

Posts: 19
Registered: 31-5-2019
Member Is Offline
|
|
I do believe, when making nitric acid the bisulphate method, salt contamination can form hydrogen chloride which will get into the nitric acid.
|
|
|
B(a)P
International Hazard
    
Posts: 1139
Registered: 29-9-2019
Member Is Offline
Mood: Festive
|
|
Boil it down until you get the first crystals then chill it and filter out fairly pure sodium nitrate.
|
|
|
j_sum1
Administrator
       
Posts: 6334
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Quote: Originally posted by B(a)P  | | Boil it down until you get the first crystals then chill it and filter out fairly pure sodium nitrate. |
NaCl
has a fairly flat solubility curve. It might not be easy to get all of it.
|
|
|
danieldavies
Harmless

Posts: 19
Registered: 31-5-2019
Member Is Offline
|
|
Is there a way to remove HCl contamination from HNO3 instead? I am just trying to recycle my chemicals.
|
|
|
B(a)P
International Hazard
    
Posts: 1139
Registered: 29-9-2019
Member Is Offline
Mood: Festive
|
|
Sorry, what I meant was to make use of that flat solubility curve. I also made the assumption that the OP had mostly sodium nitrate in solution. If
the solution is boiled down until crystals first form then cooled the vast majority of the precipitate should be sodium nitrate, which can be filtered
out
|
|
|
Bedlasky
International Hazard
    
Posts: 1243
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
HNO3 reacts with HCl to form nitrosyl chloride and chlorine.
|
|
|
danieldavies
Harmless

Posts: 19
Registered: 31-5-2019
Member Is Offline
|
|
Are you saying any HCl contamination will gas off as nitrosyl chloride and chlorine?
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Chloride and nitrate can't be easily separated in an highly acid environment like this. The nitrosyl makes that impossible. You can't gas of nitrosyl
chloride easily, unless you boil down extensively.
Edit: when boiling down nitrosyl chloride, part of it will decompose to a chloride, leaving the chloride behind in solution. Unless you use a big
excess of nitrate.
[Edited on 12-10-2021 by Tsjerk]
|
|
|
danieldavies
Harmless

Posts: 19
Registered: 31-5-2019
Member Is Offline
|
|
All i want, is to keep chloride contamination out of my nitric acid.
|
|
|
B(a)P
International Hazard
    
Posts: 1139
Registered: 29-9-2019
Member Is Offline
Mood: Festive
|
|
Then use the flat solubility curve of sodium chloride to precipitate your sodium nitrate.
|
|
|
danieldavies
Harmless

Posts: 19
Registered: 31-5-2019
Member Is Offline
|
|
Can you explain a bit more please?
|
|
|
B(a)P
International Hazard
    
Posts: 1139
Registered: 29-9-2019
Member Is Offline
Mood: Festive
|
|
Presumably you know how much sodium chloride you have? The solubility of sodium chloride is largely uneffected by temp. Boil your solution down until
you have just a little more than would dissolve your quantity of sodium chloride. Then chill the solution down to around 0C and sodium nitrate will
precipitate out. You can do a recrystallisation to improve purity
|
|
|
danieldavies
Harmless

Posts: 19
Registered: 31-5-2019
Member Is Offline
|
|
I don't have a clue on how much sodium chloride is in the solution.
|
|
|
B(a)P
International Hazard
    
Posts: 1139
Registered: 29-9-2019
Member Is Offline
Mood: Festive
|
|
Did you weigh any of your reagents? Do you have a feel for how much excess sodium chloride was added? If so go from there, if not boil untill you see
your first crystals then chill and filter off your sodium nitrate. Using the latter method you may have significant chloride contamination so a
recrystallisation is definitely needed.
|
|
|
danieldavies
Harmless

Posts: 19
Registered: 31-5-2019
Member Is Offline
|
|
Ratio is around 10/1. So 10% sodium contamination at the most. Would a 3rd recrystallisation be of any benefit?
|
|
|
macckone
Dispenser of practical lab wisdom
    
Posts: 2168
Registered: 1-3-2013
Location: Over a mile high
Member Is Offline
Mood: Electrical
|
|
Separating sodium chloride and nitrate using water is going to be difficult. Lets look at a solubility graph to understand why.
Keeping in mind common ion effects make this even more complicated.
if you dissolve it in minimum boiling water then allow to cool you should get almost pure sodium nitrate with 50% recovery.
Depending on the purity to you need, it may take a couple of rounds. The second crystallization should be pure enough for most purposes, certainly
making nitric acid.
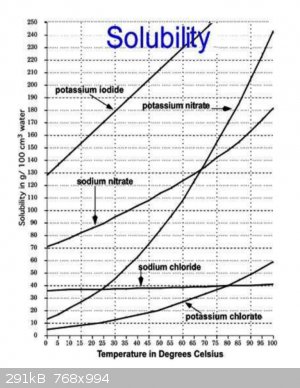
|
|
|
teodor
National Hazard
   
Posts: 924
Registered: 28-6-2019
Location: Netherlands
Member Is Offline
|
|
You can try to distill the mixture of HNO3 and HCl with Pb(NO3)2. I suppose it will precipitate chloride ions as PbCl2. My assumption is based on the
fact that distilling HNO3 with AgNO3 is the method of getting chloride-free HNO3. Pb is just much cheaper. But for complete removal, you can try the
second run with AgNO3.
Edit.
Of course, as the first step you should add Pb(NO3)2 to your mixture of NaNO3 and NaCl and filter, but a small percent of chloride will stay in the
solution (PbCl2 is slightly soluble). Filter it after cooling, it will allow precipitate more chloride.
Update.
PbCl2 you can convert to Pb(OH)2 with NaOH and then use HNO3 to regenerate Pb(NO3)2.
In a case when you have too much NaCl in the starting mixture (which makes no sense of the above procedure) you can try to melt the salt mix. I
suppose NaCl will go to the separate layer because its melting temperature is MUCH higher.
[Edited on 13-10-2021 by teodor]
|
|
|
Fulmen
International Hazard
    
Posts: 1725
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
Wouldn't the common ion effect work to our advantage here? The highly soluble nitrate ion should depress the solubility of the chloride further. So
boiling it down should precipitate NaCl first fairly exclusively.
We're not banging rocks together here. We know how to put a man back together.
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Quote: Originally posted by Fulmen  | | Wouldn't the common ion effect work to our advantage here? The highly soluble nitrate ion should depress the solubility of the chloride further. So
boiling it down should precipitate NaCl first fairly exclusively. |
Probably
|
|
|
macckone
Dispenser of practical lab wisdom
    
Posts: 2168
Registered: 1-3-2013
Location: Over a mile high
Member Is Offline
Mood: Electrical
|
|
Fulmen,
With only 10% NaCl, he would get nitrate precipitating first.
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Maybe you should clarify here: 10 NaNO3 or 10 NaCl?
|
|
|
papaya
National Hazard
   
Posts: 615
Registered: 4-4-2013
Member Is Offline
Mood: reactive
|
|
I have a similar problem, since once in the past I've had bought a NaNO3 reagent that turned out to be adulterated with significant amount of chloride
(why ???). I've researched a little bit into this problem, read some literature, and it turns out that this particular System is indeed difficult to
separate but NOT IMPOSSIBLE. It is described that if a saturated hot solution (by both salts) is cooled down sodium nitrate is precipitaed mostly,
while some amount of chloride even can be dissolved back during the process, i.e. chloride forces out nitrate! Also it is known that NaCL solubility
is nearly temperature independent! Combining these two facts there is a route for seperation : FIRST we boil down the mixed solution untill
significant amounts of precipitate forms (NACL !!!) in still BOILING solution, which is quickly hot filtered and thrown away. SECOND: the filtrate is
cooled down to precipitate the NaNO3 this time! Now nitrate is collected and the filtrate sent back to step one (repeated). I've done this myself once
for a test and it really worked, but due to much labour I've not continued it to the whole batch. Please report back if you try this yourself. EDIT:
This should work well for depleted mixes, rather than something like 90+% nitrate, in that case you will probably loose some nitrate alongside with
chloride in the step one. however once your solution gets "depleted"(saturated by both salts?) then you can continue with this method.
[Edited on 13-10-2021 by papaya]
|
|
|
Fulmen
International Hazard
    
Posts: 1725
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
@macckone: I think papaya is right.
First boil it down until most of the chloride is precipitated. The high solubility of the nitrate should depress the chloride solubility at this
point.
Then cool to precipitate the nitrate. As this decreases the sodium concentration the solubility of NaCl should increase (or at least drop less than it
normally would), increasing separation.
We're not banging rocks together here. We know how to put a man back together.
|
|
|
| Pages:
1
2 |