Lion850
National Hazard
   
Posts: 517
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Attempt to make magnesium chromate
I thought a colored magnesium salt would be cool, and was keen to do something with my chromium trioxide so decided to give magnesium chromate a go.
Looking online most references mentions MgCrO4 as being yellow, but sometimes orange or orange brown. An image search brings up yellow, orange, and
orange-brown photos. And there are photos that shows magnesium dichromate as yellow! I don't think some of the photos from Indian supplier websites is
completely accurate.
From reading articles online it is clear that both magnesium chromate and dichromate exists in various hydrates, the pentahydrate being the most
common from what I can gather. The higher hydrates usually loose some water under 100C, the pentahydrate is stable until well over 100C, and the
compounds themselves don't decompose until several 100C. So it seemed feasible to dry the salt on a steambath.
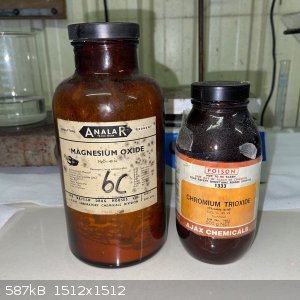
My starting materials were CrO3 and MgO. Both old chemicals, with the CrO3 being slightly damp and the MgO seemingly to have partially converted to
carbonate; see below. For the stoichiometry I simply assumed pure chemicals.
- 20g CrO3 added to a small beaker with 30g water, this was then stirred until fully desolved.
- 8.1g of MgO was measured out (it's fluffy and a large bulk) and slowly added to the CrO3 solution while stirring. With each addition there was a bit
of bubbling, and the solution got quite hot.

- The bubbling confused me and I then wondered whether some MgO had reacted with CO2 and converted to the carbonate. To test this I added some of the
MgO to hydrochloric acid; and it did indeed bubble a tiny bit but much less than a pure carbonate would. So I assume the MgO did contain carbonate but
not a lot.
- By the time all the MgO was added the color of the solution was red-brown and it was not far from boiling.
- I left it to stir for 2 hours. By then we was still a few tiny flakes of MgO going around and the pH was around 4; which indicated to me that most
of the chromium acid had reacted.
- The solution was then left to gravity filter overnight. The next morning this wine red filtrate was observed.

- There was a small amount of what I assume is MgO in the remainder, but note the layer of black particles on the filter paper. Can this be metallic
chromium, and if so why?

- The filtrate was then put on a steam bath. After 2 hours a few grams of weight was lost and there was a crust on the liquid.

- More weight was lost the next 2 hours, but very little, and then nearly nothing during a final 2 hours. Then it looked like this.

- I realized the steam bath was not going to dry this any more, but I also wondered whether the salt dissolved in its own water of crystallization.
The evaporating dish was left on the bench while I had to do something else.
- When I returned 2 hours later it was solid!

- It was quite dry, with a consistency of a cake of soap: The spatula struggled to penetrate it but little shavings could be scraped off. Below shows
the bigger broken up pieces with a lot of shavings already removed.

- The final recovery was 33.3g. The thin shaving are more orange-yellow, but the bulky pieces have a more orange-brown outside layer.

So what is it? The chromate or dichromate? From the color it appears it may be a mix of the two, although the below equation matches the obtained
product weight perfectly:
MgO + 2CrO3 + 5H2O = Cr2MgO7.5H2O
Of course there is now way I can get 100% yield because some bit were still left in the evaporating dish, it may not be 100% dry, could be a mix of
hydrates. So I am still inclined to think it is a mix of chromate and dichromate. Your comments and thoughts will be appreciated!
I did a few very basic tests:
- I drop methanol onto a tiny bit of the product. When methanol is dropped onto my CrO3 starting material it immediately catches fire (quite
spectacular) but when dropped onto the product there is no reaction.
- I poured some concentrated HCl into the evaporating dish that still had some scraps of product; the product goes dark and then green and dissolves
in the acid with a smell of chlorine.

|
|
|
Fantasma4500
International Hazard
    
Posts: 1682
Registered: 12-12-2012
Location: Dysrope (aka europe)
Member Is Offline
Mood: dangerously practical
|
|
colorful chemicals should always come with a taste-rating.
|
|
|
unionised
International Hazard
    
Posts: 5135
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
The dark stuff on the filter is probably something like Chromium (III) chromate (VI)
An interesting outcome would be
https://en.wikipedia.org/wiki/Chromium(IV)_oxide
Wash it with plenty of water, then se if it is magnetic.
[Edited on 19-9-21 by unionised]
|
|
|
woelen
Super Administrator
        
Posts: 8082
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I have reagent grade magnsium chromate. It has a golden yellow color and is quite soluble in water. It certainly is not as orange as the material you
end up with. I have the impression that your product is a mix of the chromate and dichromate.
|
|
|
chloric1
International Hazard
    
Posts: 1159
Registered: 8-10-2003
Location: GroupVII of the periodic table
Member Is Offline
Mood: Stoichiometrically Balanced
|
|
pH too low
Pretty sure you mentioned a pH of 4.1. The dichromate ion begins to form at pH of 3-4. I’d think you need to raise pH at least to 6 to have all
chromate, maybe even a pH beyond 7.
Fellow molecular manipulator
|
|
|
Admagistr
Hazard to Others
  
Posts: 383
Registered: 4-11-2021
Location: Central Europe
Member Is Offline
Mood: The dreaming alchemist
|
|
I once made MgCrO4 from MgO, distilled water and CrO3, it was yellow! Definitely not orange! And the MgO dissolved well, no problem, the problem came
later when I boiled it to force it to crystallize, with a water droplet trap, it was a ground glass part, there was an accident, an explosion due to
the overpressure of the water vapour and the chromate splashed all over the room, then I wiped the toxic droplets all over the room and there was
always another yellow spot...Thus the unpleasant ending and I lost all the yield...
|
|
|