Yttrium2
Perpetual Question Machine
    
Posts: 1104
Registered: 7-2-2015
Member Is Offline
|
|
Solar stills
How well would a large plastic garbage back on the roof, with a tube coming off of it work for a solar still?
Idea is kind of like those camping shower bags, but inverted, so that the vapors come up through the tube and condense.
I’m still wondering what the best way is to distill around 300mL of naphtha is.
I’m on a budget, and figured a solar still would be up my ally.
Please don’t recommend laboratory glassware, as I am looking for some ghetto, by this I mean innovative, not with expensive setups, something DIY.
Wondering if anyone has anything wiser / cheaper than a black garbage back in the sun.
|
|
|
Texium
Administrator
       
Posts: 4580
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
You aren’t gonna distill naphtha in anything plastic. Especially not garbage bags. End of story.
|
|
|
macckone
Dispenser of practical lab wisdom
    
Posts: 2168
Registered: 1-3-2013
Location: Over a mile high
Member Is Offline
Mood: Electrical
|
|
copper is going to work better for naphtha.
You can also use black steel.
You can get prethreaded pieces and then use a copper condensor with water cooling.
You could do it as a solar still with a larger steel container and a cooled (shaded) portion with a tube.
You might be able to get away with polyethylene tubing but it will degrade quickly.
Other options are a mason jar and polyethylene tubing.
You can also use beer bottles with some teflon tape to seal a piece of copper tubing to the bottle.
A dark beer bottle might be just the ticket.
|
|
|
Yttrium2
Perpetual Question Machine
    
Posts: 1104
Registered: 7-2-2015
Member Is Offline
|
|
How’s this for a condenser? It’s 1/4” Outer Diameter, so they say.. At 25foot long.
It won’t fit snug in my bungs stoppers acquired from local home brew supply / brewery , they require, or are meant for 3/8 diameter air lock
fittings, it’s also the white gum stopper which is less ideal for chemistry than the black stoppers, (maybe one day I’ll know why?)
Why is it hard to blow air through — the tubing (PVC)
Is there straight PVC tube like this?
If I were to boil water in a 100 ft tubing the vapor would go as far as — depending on the heat of the boil? *EDIT (the volume of hot vapor emitted)
And the specific heat/cooling capacity of the material of tubing used as a condenser right? Or would the heat creep along the condenser, pushing the
condensation point of the condenser further and further until steam comes out the other end?
I forget why I’m asking this.
3/8 OD PVC would be ideal, I tried to straighten the hose and remove the coil but it just untangled the kinks I had somehow managed to induce.
I’m willing to bet the pvc will be adequate at such a length, knowing nothing about specific heat or the cooling capacity’s of the materials
(copper and pvc) and having done no experimentation of my own.
It was done entirely through inductive reasoning.
Also
I’m thinking I can drop the coil through a 2-3 liter bottle with a hole at the bottom, and run a bit of tubing through it, so that it will function
as a Graham condenser? The narrow passage of the tubing may make it unsuitable for reflux, as well as the coil configuration inside of a bottle -
That’d be a Graham condenser, not a reflux condenser… Anyone care to chime in as to why a graham condenser is unsuitable for reflux of vapors? It
seems to me that it would have more cooling area than a reflux condenser.

[Edited on 9/6/2021 by Yttrium2]
|
|
|
Yttrium2
Perpetual Question Machine
    
Posts: 1104
Registered: 7-2-2015
Member Is Offline
|
|
3 liter bottle…
[Edited on 9/6/2021 by Yttrium2]

|
|
|
Yttrium2
Perpetual Question Machine
    
Posts: 1104
Registered: 7-2-2015
Member Is Offline
|
|


|
|
|
macckone
Dispenser of practical lab wisdom
    
Posts: 2168
Registered: 1-3-2013
Location: Over a mile high
Member Is Offline
Mood: Electrical
|
|
Copper tubing comes in a lot of sizes.
For a condenser tube you probably want something bigger to prevent too much back pressure which will blow out a bung.
The issue with polyethylene tubing is that it isn't stable in hot organic solvents, especially saturated hydrocarbons.
1/2" copper tubing:
https://www.amazon.com/Copper-Soft-Type-Refrigeration-Tubing...
3/8" copper tubing:
https://www.homedepot.com/p/Everbilt-3-8-in-x-20-ft-Soft-Cop...
and of course you are familiar with 1/4" (which is probably too small).
You can cut a rubber bung to the correct diameter opening with a drill or cutter.
Cork cutters are less than ideal as they aren't designed for rubber.
Basically the same as what you have but better materials.
Glass (glass jug of some kind), use teflon tape to make a bung, and copper tube through your water bath.
|
|
|
Twospoons
International Hazard
    
Posts: 1324
Registered: 26-7-2004
Location: Middle Earth
Member Is Offline
Mood: A trace of hope...
|
|
You're still thinking in terms of a lab still. Your solar still is going to be much slower, as the heat input will be quite small. So keep it
simple: one metal can in the sun, one in the shade, connected by a short fat pipe and both painted black. A single layer of clear bubble wrap (smooth
side out) on the hot can will improve things by reducing convection heat loss.
[Edited on 7-9-2021 by Twospoons]
Helicopter: "helico" -> spiral, "pter" -> with wings
|
|
|
Twospoons
International Hazard
    
Posts: 1324
Registered: 26-7-2004
Location: Middle Earth
Member Is Offline
Mood: A trace of hope...
|
|

Further improvement would be to make the wall silver with aluminium foil, to reflect more sun on the hot can, and keep the shady side of the wall
cooler by preventing the wall from heating up.
[Edited on 8-9-2021 by Twospoons]
Helicopter: "helico" -> spiral, "pter" -> with wings
|
|
|
violet sin
International Hazard
    
Posts: 1480
Registered: 2-9-2012
Location: Daydreaming of uraninite...
Member Is Offline
Mood: Good
|
|
Enclose the styrofoam wall around the receiver?
Ice chest, cheap styrofoam kind ~3$ at any gas station and dollar store aluminum foil would be quick to source and cheap,
Add a little ice in the chest, on a warm day in so•cal, bet it works faster than you'd imagine on 100ml scale. Don't think the ice chest would like
leaks much though... The insulated wall idea would provide more draft if it pressurized in bright sun, and vented at seals.
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Although a setup like this would work for e.g. water, I don't think it would for something volatile like naphtha.
[Edited on 8-9-2021 by Tsjerk]
|
|
|
Herr Haber
International Hazard
    
Posts: 1236
Registered: 29-1-2016
Member Is Offline
Mood: No Mood
|
|
A PVC condenser ?
The condenser is a heat exchanger. You're not doing yourself any favor by chosing a poor thermal conductor.
The spirit of adventure was upon me. Having nitric acid and copper, I had only to learn what the words 'act upon' meant. - Ira Remsen
|
|
|
macckone
Dispenser of practical lab wisdom
    
Posts: 2168
Registered: 1-3-2013
Location: Over a mile high
Member Is Offline
Mood: Electrical
|
|
tsjerk,
naphtha is a mixture and different naphtas have different boiling points and component profiles.
some components may be volatile, some may be less volatile than water.
too many variables, cooling the receiver and condensor with ice will solve a lot of volatility problems.
|
|
|
macckone
Dispenser of practical lab wisdom
    
Posts: 2168
Registered: 1-3-2013
Location: Over a mile high
Member Is Offline
Mood: Electrical
|
|
I agree with Herr Haber, PVC or polyethylene is going to be poor choice for distilling naphtha in addition to the poor conductivity.
|
|
|
Yttrium2
Perpetual Question Machine
    
Posts: 1104
Registered: 7-2-2015
Member Is Offline
|
|
 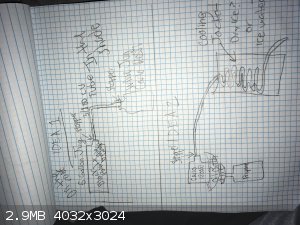 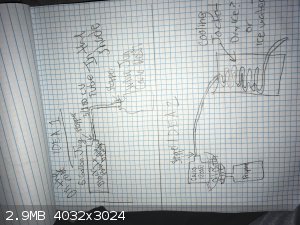
|
|
|
macckone
Dispenser of practical lab wisdom
    
Posts: 2168
Registered: 1-3-2013
Location: Over a mile high
Member Is Offline
Mood: Electrical
|
|
as ideas, all of those look doable.
the key things are going to be suitable materials, control of volatility and heat transfer.
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Quote: Originally posted by macckone  | tsjerk,
naphtha is a mixture and different naphtas have different boiling points and component profiles.
some components may be volatile, some may be less volatile than water.
too many variables, cooling the receiver and condensor with ice will solve a lot of volatility problems. |
I have the idea I know what kind of naphtha Yttrium2 is taking about, and it is the lighter fuel one. That is distilled to contain only the volatile
part.
|
|
|
MadHatter
International Hazard
    
Posts: 1339
Registered: 9-7-2004
Location: Maine
Member Is Offline
Mood: Enjoying retirement
|
|
Reflux
Yttrium2, using a coil(Graham) condenser could be problematic
trying to get the vapors to travel up the coils. I'm thinking
resistance. OTOH, in a dimroth condenser the coolant flows
through the coils and would give plenty of surface area for
condensation. This is the only explanation I can come up with.
From opening of NCIS New Orleans - It goes a BOOM ! BOOM ! BOOM ! MUHAHAHAHAHAHAHA !
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
Quote: Originally posted by Tsjerk  | Quote: Originally posted by macckone  | tsjerk,
naphtha is a mixture and different naphtas have different boiling points and component profiles.
some components may be volatile, some may be less volatile than water.
too many variables, cooling the receiver and condensor with ice will solve a lot of volatility problems. |
I have the idea I know what kind of naphtha Yttrium2 is taking about, and it is the lighter fuel one. That is distilled to contain only the volatile
part. |
Yeah and I think this stuff suits his...
What was anyone else thinking actually he was about to do with it? 
I was about to say, I think this stuff suits his meth synthesis attempt already like it is.
It is more important that he builds himself a distillation apparatus which is able to handle hot steam.
Because fuck that naphtha, you're not going to drink it.
But you plan on imbibing the produced meth.
At least steam distill it, as what you are about to do is as far as the forensic literature suggest, extraordinary dirty.
And this is about your health, you should have put all the energy in this foremost, in my opinion at least.
verrückt und wissenschaftlich
|
|
|