| Pages:
1
2 |
Romix
Hazard to Others (Literally)
  
Posts: 483
Registered: 19-6-2015
Member Is Offline
Mood: No Mood
|
|
Few questions about Gold recovery
Here is gold after washing it with 20 litters of water.

|
|
|
Romix
Hazard to Others (Literally)
  
Posts: 483
Registered: 19-6-2015
Member Is Offline
Mood: No Mood
|
|
No matter how much I wash it, solution still dirty. Why ?

|
|
|
Romix
Hazard to Others (Literally)
  
Posts: 483
Registered: 19-6-2015
Member Is Offline
Mood: No Mood
|
|
And after dropping the Gold with Sodium Sulfite, I added KOH to the solution that's what precipitated. Any ideas what is it?
Solution still were lightly greenish after filtering it.
I've checked PH, basic.

[Edited on 17-6-2021 by Romix]
[Edited on 17-6-2021 by Romix]
[Edited on 18-6-2021 by Romix]
[Edited on 18-6-2021 by Romix]
|
|
|
paulll
Hazard to Others
  
Posts: 112
Registered: 1-5-2018
Member Is Offline
Mood: It's fine. Really.
|
|
Any chance there's a bunch of iron in there?
|
|
|
Romix
Hazard to Others (Literally)
  
Posts: 483
Registered: 19-6-2015
Member Is Offline
Mood: No Mood
|
|
Could be, I dissolved 8 ceramic processors + a lot of other gold containing scrap. Here's just 200ml tester.
|
|
|
Romix
Hazard to Others (Literally)
  
Posts: 483
Registered: 19-6-2015
Member Is Offline
Mood: No Mood
|
|
That's how pure gold looks like, dropped it from dissolved Alaskan nuggets.
[Edited on 17-6-2021 by Romix]

|
|
|
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
Did U just aqua regia the straight processors without first dissolving tramp metals?
Could you just wash the red goo with nitric acid to remove some of the crap?
|
|
|
paulll
Hazard to Others
  
Posts: 112
Registered: 1-5-2018
Member Is Offline
Mood: It's fine. Really.
|
|
Ooh that looks like it'd be satisfying to melt down.
I think you're carrying a lot of Iron and Nickel; I'd decant that off and treat the precipitate with successive HCl washes 'til it comes up clear, and
take it from there.
|
|
|
Romix
Hazard to Others (Literally)
  
Posts: 483
Registered: 19-6-2015
Member Is Offline
Mood: No Mood
|
|
I tried boiling it in 3% nitric first... but after an hour nothing happened.
So chucked in very diluted AR.
Yes, that's what I thought about doing. Just waiting for the wash solutions to settle down. And they still dirty after whole day standing.
|
|
|
paulll
Hazard to Others
  
Posts: 112
Registered: 1-5-2018
Member Is Offline
Mood: It's fine. Really.
|
|
Quote: Originally posted by Romix  | I tried boiling it in 3% nitric first... but after an hour nothing happened.
So chucked in very diluted AR.
Yes, that's what I thought about doing. Just waiting for the wash solutions to settle down. And they still dirty after whole day standing.
|
It'll probably take a few goes-around but I'd put aside the nitric (Actually if I suspected there was any there I'd wash it out like a lipstick stain)
and AR, and focus on dissolving everything that *isn't* Gold.
|
|
|
Romix
Hazard to Others (Literally)
  
Posts: 483
Registered: 19-6-2015
Member Is Offline
Mood: No Mood
|
|
After boiling it in water, solution became clearer!
|
|
|
Romix
Hazard to Others (Literally)
  
Posts: 483
Registered: 19-6-2015
Member Is Offline
Mood: No Mood
|
|
Added nitric all dissolved!!!!!! No gold left!!!!
[Edited on 18-6-2021 by Romix]
|
|
|
Romix
Hazard to Others (Literally)
  
Posts: 483
Registered: 19-6-2015
Member Is Offline
Mood: No Mood
|
|
Solution is green.
|
|
|
Romix
Hazard to Others (Literally)
  
Posts: 483
Registered: 19-6-2015
Member Is Offline
Mood: No Mood
|
|
200ml lost, what to do with the rest of gold containing solution?
|
|
|
Romix
Hazard to Others (Literally)
  
Posts: 483
Registered: 19-6-2015
Member Is Offline
Mood: No Mood
|
|
It took few seconds for all of it to dissolve, reaction was exothermic.
|
|
|
Romix
Hazard to Others (Literally)
  
Posts: 483
Registered: 19-6-2015
Member Is Offline
Mood: No Mood
|
|
Google saying that this yellow - orange precipitate is CuOH.
[Edited on 20-6-2021 by Romix]

|
|
|
Romix
Hazard to Others (Literally)
  
Posts: 483
Registered: 19-6-2015
Member Is Offline
Mood: No Mood
|
|
Could be, decomposed it to black oxide and flame test is green.
[Edited on 20-6-2021 by Romix]

|
|
|
Romix
Hazard to Others (Literally)
  
Posts: 483
Registered: 19-6-2015
Member Is Offline
Mood: No Mood
|
|
Cu(OH)2
[Edited on 21-6-2021 by Romix]

|
|
|
Romix
Hazard to Others (Literally)
  
Posts: 483
Registered: 19-6-2015
Member Is Offline
Mood: No Mood
|
|
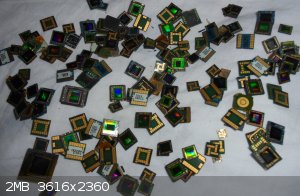
That's camera lenses of the phone's that I've dissolved, even double that because half of them didn't had cameras, were to old.
+ 8 ceramic processors.
Please help, what to drop gold with?
[Edited on 23-6-2021 by Romix]
[Edited on 23-6-2021 by Romix]
|
|
|
Romix
Hazard to Others (Literally)
  
Posts: 483
Registered: 19-6-2015
Member Is Offline
Mood: No Mood
|
|
Lenses only weigh 15 grams. I reckon 1/100 of the weight gold maybe more.
[Edited on 23-6-2021 by Romix]

|
|
|
Romix
Hazard to Others (Literally)
  
Posts: 483
Registered: 19-6-2015
Member Is Offline
Mood: No Mood
|
|
Only neutralized wash waters of copper nitrate solutions. Do you know maybe phone PCB's also contain nickel?
Still have plenty of concentrate.
Found cheap funnel on Ebay 4.5 litters.
Will neutralize all metals with KOH, filter it out, leave evaporate through the summer and then I'll try to crystalize KNO3.
Tried feeding KNO3 from wash solutions to my garden flowers, they died next day after watering.
And then dry dirty Cu(OH)2 on my LED lamp.
[Edited on 23-6-2021 by Romix]

|
|
|
Romix
Hazard to Others (Literally)
  
Posts: 483
Registered: 19-6-2015
Member Is Offline
Mood: No Mood
|
|
+ All phones yielded bit over 200 grams of gold plated pins.
[Edited on 23-6-2021 by Romix]

[Edited on 23-6-2021 by Romix]
|
|
|
Romix
Hazard to Others (Literally)
  
Posts: 483
Registered: 19-6-2015
Member Is Offline
Mood: No Mood
|
|
Ceramic lenses from old digital cameras, under 4 megapixels.
Maybe someone have a small device to crash them, interested in buying one.
They'll break average kitchen blender.
[Edited on 23-6-2021 by Romix]

|
|
|
Romix
Hazard to Others (Literally)
  
Posts: 483
Registered: 19-6-2015
Member Is Offline
Mood: No Mood
|
|
Tried cementing sample on copper.
Small amount of black mud formed, I can tell by the look of it that it's not metal.
And all mud dissolved in nitric.
There was some metal but all of it stuck to the walls of a beaker.
After few days copper bar passivated with white CuCl and recovery stopped.
And a lot of insoluble salt farmed on a top of the solution, when AR was very very diluted.
[Edited on 24-6-2021 by Romix]

[Edited on 24-6-2021 by Romix]
|
|
|
Romix
Hazard to Others (Literally)
  
Posts: 483
Registered: 19-6-2015
Member Is Offline
Mood: No Mood
|
|
Sodium Metabisulfite. After night standing some mirror like dirt appeared on a beaker's walls.
Added a lot of SMB, copper salts not precipitating like it was with NaSO3.
[Edited on 27-6-2021 by Romix]

[Edited on 27-6-2021 by Romix]
[Edited on 27-6-2021 by Romix]
|
|
|
| Pages:
1
2 |