chemist1243
Hazard to Others
  
Posts: 170
Registered: 7-8-2019
Member Is Offline
|
|
N-acetyl MDA from helional
Okay, hear me out. For starters, i think we can agree that N-acetyl MDA probably isnt a “drug”. There is barely any information on its activity,
use, or just info about it in general. So if this is taken down let it be because i dont know what I’m talking about, not “drug” synthesis.
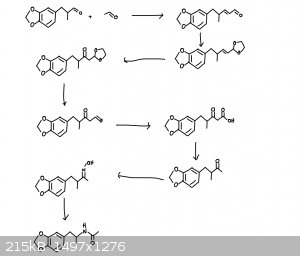
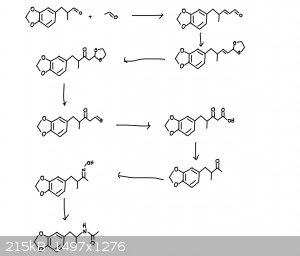
I propose a pathway from helional to N-Acetyl MDA. First, aldol condensation between helional and acetyl aldehyde. This resulting aldehyde is
converted into its acetal, and the adjacent double bond is converted to a ketone, the acetyl is then converted back to its aldehyde, the aldehyde is
oxidized to the carboxylic acid, then decarboxylation is carried out to form the methyl ketone. From there, convert this to the ketoxime and perform
the beckmann rearrangement to form N-acetyl MDA.
It should look something like the attached photo.
Please poke as many holes in my logic as possible! I have not the slightest clue what I’m doing or what would be the proper catalysts used for the
aldol and beckmann would be.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2787
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
I mean, your essential problem is simple: you're trying to obtain C-methylation of the carbonyl carbon, but you've picked a severely overcomplicated
means of doing so.
The homologation of aldehydes to methyl ketones is much easier than that. It can be done in two steps, whereas you've used five (four if
beta-keto decarboxylation is considered spontaneous).
One possible rxn system uses methyl iodide and manganese powder in THF to give MeMnI, which reacts with helional to the secondary alcohol. Oxidation
gives your sixth intermediate. Suitably activated Mn suspensions are usually generated by rxn of a solution of MnCl2 with lithium naphthalenide or
similar radical anions.
I don't feel that you've posed the problem in a particularly good way with regard to the rules. While N-acetyl MDA is not a listed substance, everyone
knows what it represents. But you seem to be focused mostly on the interesting chemistry problem of aldehyde homologation to a methyl ketone,
which is a valid topic of discussion.
What we don't like is spoonfeeding, "how do I make X". What we like is chemistry, "how do I accomplish transformation Y". The first kind of threads
not only attract unwanted attention but can quickly devolve into endless empty speculation about other possible routes to X, with only superficial
discussion of the actual chemistry. That's not good under any legal system.
|
|
|
chemist1243
Hazard to Others
  
Posts: 170
Registered: 7-8-2019
Member Is Offline
|
|
Yeah, there are myriad of better ways go about this. I would never go through the hell of this synthesis, but as it is one of the few complex
synthesis schemes I composed myself, I’m curious to know if my logic is right. All theory.
Ive thought about the Grignard, but thats rather simple and i know it back and fourth, so it seemed boring.
Speaking of ketones, I need a chemistry genie to lend me some CuCl2 and morpholine, I got the O2 covered!
|
|
|
Metacelsus
International Hazard
    
Posts: 2539
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
Yeah, even a methyl Grignard would probably work to homologate it.
edit: beat me to it
[Edited on 2021-5-13 by Metacelsus]
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2787
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
How do you expect the double bond will be converted to a ketone? I'm not convinced that you would have effective regioselectivity -- why wouldn't the
carbonyl end up on the other carbon, alpha to the acetal? The carbons are near-identical.
|
|
|
chemist1243
Hazard to Others
  
Posts: 170
Registered: 7-8-2019
Member Is Offline
|
|
Yeah, another issue with this scheme.
I believe on phenyl propenes, when water leaves from the benzylic carbon, the resulting carbocation is stablized by the adjacent benzene ring, the
hydroxyl group that stays is on the 2 carbon and becomes ketone. Because wouldnt it be a less stable system with the cation further away from the
ring? I’m not positive but i think thats right.
But with the double bond in my scheme, i dont think there is that same selectivity, and really i could see it going either way as well. The only thing
that could stablize one of the carbocations over the other is maybe the acetal’s oxygen(s), but they aren’t close enough to either of the carbons
for resonance anyways.
|
|
|
chemist1243
Hazard to Others
  
Posts: 170
Registered: 7-8-2019
Member Is Offline
|
|
Yeah, another issue with this scheme.
I believe on phenyl propenes, when water leaves from the benzylic carbon, the resulting carbocation is stablized by the adjacent benzene ring, the
hydroxyl group that stays is on the 2 carbon and becomes ketone. Because wouldnt it be a less stable system with the cation further away from the
ring? I’m not positive but i think thats right.
But with the double bond in my scheme, i dont think there is that same selectivity, and really i could see it going either way as well. The only thing
that could stablize one of the carbocations over the other is maybe the acetal’s oxygen(s), but they aren’t close enough to either of the carbons
for resonance anyways.
|
|
|
njl
National Hazard
   
Posts: 609
Registered: 26-11-2019
Location: under the sycamore tree
Member Is Offline
Mood: ambivalent
|
|
Consider the schmidt reaction of HN3 with carbonyls, which under some conditions yields formamides from aldehydes. The products with aldehydes are
usually messy, yielding the nitrile, formamide, amide, and acid. I'm sure there are ways to optimize the reaction to yield the formamide. However, the
schmidt reaction of HN3 with carboxylic acid invariably yields the amine.
Reflux condenser?? I barely know her!
|
|
|
chemist1243
Hazard to Others
  
Posts: 170
Registered: 7-8-2019
Member Is Offline
|
|
Yeah, I’ll have to pick up a few canisters.
I joke, i joke
|
|
|
AvBaeyer
National Hazard
   
Posts: 651
Registered: 25-2-2014
Location: CA
Member Is Offline
Mood: No Mood
|
|
Way too much unreliable or unknown chemistry along with inefficient use of carbon atoms. Make the oxime of the aldehyde, transform that to the
nitrile, then to the amide and rearrange to the amine. There are multiple proven routes for each step. Easy to find.
AvB
|
|
|
njl
National Hazard
   
Posts: 609
Registered: 26-11-2019
Location: under the sycamore tree
Member Is Offline
Mood: ambivalent
|
|
Since it's back on my mind, maybe the Schmidt reaction can be optimized for the synthesis of formamides from aldehydes. According to the mechanism
given in "The Schmidt reaction" (linked below), the pathway leading to the nitrile requires an intermediate diazoiminium compound to take the trans
conformation, while the cis conformation gives the formamide. Once N2 leaves that intermediate, it will either rearrange, hydrate, and
tautomerize to the formamide or be deprotonated to the nitrile depending on its form. I have no idea if there is a way to selectively generate either
the cis or trans form. If the mechanism given is accurate then it seems like this would be key to a higher yield of the formamide. The only other
optimization one could easily make might be an aqueous solvent system, which would allow the hydration of the formamide intermediate to proceed and
concentrated acid would hopefully make deprotonation less favorable. I haven't had much luck finding conditions for the reaction.
Attachment: SM Schmidt reaction.pdf (2.9MB)
This file has been downloaded 607 times
[Edited on 5-17-2021 by njl]
Reflux condenser?? I barely know her!
|
|
|
Opylation
Hazard to Others
  
Posts: 131
Registered: 30-8-2019
Member Is Offline
|
|
Check this paper out. It uses piperonal as the starting material but an intermediary chemical is helional.
Alternative route from what the below paper provides is oxidation to the acid, a-methyl-MD-phenylpropionic acid, react with ammonia, a lewis acid, and
molecular sieves and heat to form the amide, and then perform a Hofmann Rearrangement with bleach. I think that would be easier than trying to make
the acyl chloride to isocyanate
Man, I've edited this post about half a dozen times. But i think there's an even easier reaction where you can condense formamide with aldehydes to
get the amide. So you may even be able to skip the oxidation step
Attachment: schulze2010.pdf (352kB)
This file has been downloaded 710 times
[Edited on 19-5-2021 by Opylation]
[Edited on 19-5-2021 by Opylation]
|
|
|
njl
National Hazard
   
Posts: 609
Registered: 26-11-2019
Location: under the sycamore tree
Member Is Offline
Mood: ambivalent
|
|
If you can back up that formamide route then right on. However, if you have the acid and want the amine then the Schmidt reaction cuts out a step from
the Curtius. Also, I think you mean phenyl propionic acid.
Reflux condenser?? I barely know her!
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2787
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
In the paper Opylation posted, helional is not prepared from piperonal, for anyone who was confused as to how this might be
practical. Instead, helionoic acid is prepared from either piperonal or helional, then converted to the acyl chloride and azide by the usual methods.
The direct azidation of aldehydes remains elusive.
|
|
|
Opylation
Hazard to Others
  
Posts: 131
Registered: 30-8-2019
Member Is Offline
|
|
[Edit]: seems you’re right, not atara. It’s been a while since I read the paper and briefly going through it before posting I missed that the
starting material is heliotropin or helional. But the acid is not reduced to the aldehyde. Guess that’s what I get for just looking at the reaction
scheme instead of rereading the paper
In response to njl, I did perform a brief search last night for a reference to the aldehyde formamide condensation but didn’t look to deep into it.
I’ll go ahead an post the paper about it here. It’s dated (from the 40’s) so may or may not be a viable procedure.
| Quote: |
Also, I think you mean phenyl propionic acid
|
Ah yes! Rookie mistake
Here’s a link to a one-pot aldehyde to amide reaction
https://pubs.acs.org/doi/10.1021/ol302175v
Attachment: phpgLQKB5 (364kB)
This file has been downloaded 461 times
[Edited on 20-5-2021 by Opylation]
|
|
|
vannylaholic
Harmless

Posts: 20
Registered: 3-7-2017
Member Is Offline
Mood: inquisitive
|
|
This is way to complicated, I'm all for doing things that are novel or unherd of , but this has been done and IMHO there is way to much drug
manufacture B's on this forum
It's people that make shit like this that gives us honestly curious amateur chemist a bad name and worse stigma .
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2787
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
If you're sick and tired of drug manufacture BS, don't bump drug manufacture BS from nearly a year ago 
[Edited on 4-2-2022 by clearly_not_atara]
|
|
|
chemist1243
Hazard to Others
  
Posts: 170
Registered: 7-8-2019
Member Is Offline
|
|
Quote: Originally posted by vannylaholic  | This is way to complicated, I'm all for doing things that are novel or unherd of , but this has been done and IMHO there is way to much drug
manufacture B's on this forum
It's people that make shit like this that gives us honestly curious amateur chemist a bad name and worse stigma . |
I agree
|
|
|
Texium
Administrator
       
Posts: 4580
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Ah, lovely, so we’re all in agreement and I can close this thread.
|
|
|
Texium
|
Thread Closed
14-2-2022 at 07:58 |