itsallgoodjames
Hazard to Others
  
Posts: 276
Registered: 31-8-2020
Location: America Lite
Member Is Offline
|
|
Removing dissolved organics from ammonia solution
Yesterday I noticed that my bottle of ammonia had turned white and become stiff. I transferred to to a glass bottle. But the issue now is that the
ammonia contains some of the dissolved plastic. How would I remove the plastic contaminants from the ammonia? Obviously I can't just distill it, as
the ammonia will all come out of solution before the water boils, so how else would I purify it? Or is it just not worth it, and I should throw it
out and make some new ammonia from fertilizer?
Thanks!
Edit - I should mention, it's about 400ml of 25% ammonia solution
[Edited on 31-3-2021 by itsallgoodjames]
Nuclear physics is neat. It's a shame it's so regulated...
Now that I think about it, that's probably a good thing. Still annoying though.
|
|
|
RustyShackleford
Hazard to Others
  
Posts: 200
Registered: 10-12-2020
Location: Northern Europe
Member Is Offline
|
|
reflux the solution, put a tube from the top of the condenser to a suckback trap and then so it bubbles into a vessel of water. The ammonia will be
driven out by the high temperature and it will redissolve in the fresh water (use aprox 300ml, ice cold)
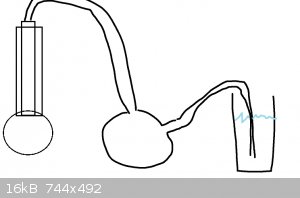
|
|
|
itsallgoodjames
Hazard to Others
  
Posts: 276
Registered: 31-8-2020
Location: America Lite
Member Is Offline
|
|
Thanks! I'll do that. So I assume I'll loose around a quarter of the ammonia, given it will redissolve in only 300ml?
Edit - wait no, I'm stupid, the ammonia makes up 25% of the volume
[Edited on 31-3-2021 by itsallgoodjames]
Nuclear physics is neat. It's a shame it's so regulated...
Now that I think about it, that's probably a good thing. Still annoying though.
|
|
|
Fulmen
International Hazard
    
Posts: 1716
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
Just use the same amount of water and you should end up with roughly the same concentration.
We're not banging rocks together here. We know how to put a man back together.
|
|
|