RogueRose
International Hazard
    
Posts: 1593
Registered: 16-6-2014
Member Is Offline
|
|
Making a scrubber to clean NO2 gas from vacuum distillation of HNO3
I've never done a vacuum distillation and I'm not very confident of distilling HNO3 to begin with, but after some fine members pointed me in the
direction of a reasonable method (starting with freezing H2SO4 + NH4NO3 under vaccum distillation), I think I can get over my fear of NO2 clouds.
The problem is that I don't have any traditional scrubber setup but I have stuff I think I can make work, I just want to make sure I have the
principles correct and am not overlooking anything.
I have some Pyrex media bottles (w/ GL45 caps) and I've altered some caps to have 2 glass tubs running through it. So I figured I could add some cold
water or H2O2 to the bottle, run the input down into the liquid and have the suction line to the pump maybe ~1" from the inside top of the cap.
I'm probably going to run an old fridge/AC/heat pump compressor so it will pull about -14.5psi, I know this is not super ideal, but I'm hoping that
will be low enough too work well.
I'm not even looking for WFNA or even RFNA, I'd be happy with azeotropic, and will be diluting to about 5-10% anyway. I just thought this might give
the best chance of little NO2 and less chance of foaming while heating.
I guess if this sounds like an adequate setup the final question is water of H2O2. I have 35% and I'm thinking it might be nice to always use this
H2O2 for HNO3 and eventually I'll have a useable quantity of acid in there.
I also have some aerators, some are quartz tube/bubbler stone and also some aquarium bubblers. Are these necessary or is the tube in the liquid
adequate?
I'm planning on using about 400g acid & 300g nitrate and doing a nice slow heat (if that's possible), I'm in no rush to finish this, just want it
done right.
|
|
|
Fyndium
International Hazard
    
Posts: 1192
Registered: 12-7-2020
Location: Not in USA
Member Is Offline
|
|
I would personally distill HNO3 under aspirator or a lab grade membrane pump. But, if I had to use rotary vane, I'm in the same pit.
But I know that much after my benzaldehyde experiment that NO2 gas don't much care about NaOH water trap with inverted funnel - it just puffed through
like nothing, so at least it would require a lot of scrubbing and aeration.
Since nitro compounds are generally safe to use with stainless steel, I wonder if one could set up a 1L flask half-full of ice cold NaOH and immerse a
tube with one of these attached that would pull the gas through as a very fine curtain of bubbles, and after that one would put coldfinger before the
pump.
Not sure, but as you mentioned H2O2, could it be mixed with NaOH to consume NO as it requires oxygen to convert to NO2 which is much more water
soluble. It could be that the gas that passed through my trap was NO, and it instantly oxidized at contact with air to form brown NO2. This was later
apparent when I used better ventilation that when I raised the funnel from the water, it instantly filled with brown gas, meaning that it was full of
NO that oxidized.

|
|
|
B(a)P
International Hazard
    
Posts: 1139
Registered: 29-9-2019
Member Is Offline
Mood: Festive
|
|
If you are looking for azeotropic nitric acid (way less than that by the sounds) I would suggest removing the vacuum from the setup and running the
distillation as you have described, but with suck back protection between your NO2 scrubber and receiver flask.
If you are happy to take your time NO2 will not be an issue in my experience. I will note that I have used KNO3 rather than AN,
so that may be a significant point of difference, hopefully others will chime in on this.
If you do want FNA or want to proceed under vacuum anyway make sure that your condenser water and receiver flask are really well cooled, as cold as
you can manage, to prevent acid vapour making it to your trap and reducing yield.
I have tried both water and 10% peroxide in a scrubber and did not find the peroxide solution to be superior.
When distilling FNA I have my condenser water as close to, if not below 0C, I cool my receiver with dry ice in acetone and I use two traps after my
receiver. The first is a side arm test tube immersed in dry ice/acetone to catch any acid vapours that make it past the receiver (it typically catches
just a few drops of FNA so is likely overkill, but I like to protect my pump and have the available equipment). The second trap is a dreschel flask
immersed in a salted ice bath about half full of dH2O.
Slow is definitely possible and advisable, particularly if you are able to have your setup out of UV light.
I usually distill about twice what you propose per batch and it takes most of the day with set up clean up ect.
Hope this helps, sounds like you are well prepared and ready to experience the joy of producing your very own nitric acid!
Edit: - options for suck back
When distilling without vacuum I use a dry dreschel flask between the receiver and scrubber, but set up in the opposite direction to the scrubber if
that makes sense, inlet has the short tube outlet has the long one.
[Edited on 24-3-2021 by B(a)P]
|
|
|
zed
International Hazard
    
Posts: 2283
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
If you aren't used to vacuum distillation, and/or trapping noxious gases. Make a practice run or two.
Things can happen. Well, actually.... Things will happen.
Always fun when you are using an aspirator pump.... Someone in the house, will flush a toilet. Water flow through your pump will be reduced. Vacuum
in your system will suddenly be greater, than that from your pump. Water will back-up from your pump. The water in your traps, will be sucked back
towards your reaction flask.
You have to have an empty flask or two in your system, and such flasks must be outfitted properly, to circumvent disasters.
That reminds me. I have some very fancy glassware now, but without a cork-borer, some erlenmeyers, stoppers, and some glass tubing... None of it is
safe to use.
|
|
|
RogueRose
International Hazard
    
Posts: 1593
Registered: 16-6-2014
Member Is Offline
|
|
Quote: Originally posted by B(a)P  | If you are looking for azeotropic nitric acid (way less than that by the sounds) I would suggest removing the vacuum from the setup and running the
distillation as you have described, but with suck back protection between your NO2 scrubber and receiver flask.
If you are happy to take your time NO2 will not be an issue in my experience. I will note that I have used KNO3 rather than AN,
so that may be a significant point of difference, hopefully others will chime in on this.
If you do want FNA or want to proceed under vacuum anyway make sure that your condenser water and receiver flask are really well cooled, as cold as
you can manage, to prevent acid vapour making it to your trap and reducing yield.
I have tried both water and 10% peroxide in a scrubber and did not find the peroxide solution to be superior.
When distilling FNA I have my condenser water as close to, if not below 0C, I cool my receiver with dry ice in acetone and I use two traps after my
receiver. The first is a side arm test tube immersed in dry ice/acetone to catch any acid vapours that make it past the receiver (it typically catches
just a few drops of FNA so is likely overkill, but I like to protect my pump and have the available equipment). The second trap is a dreschel flask
immersed in a salted ice bath about half full of dH2O.
Slow is definitely possible and advisable, particularly if you are able to have your setup out of UV light.
I usually distill about twice what you propose per batch and it takes most of the day with set up clean up ect.
Hope this helps, sounds like you are well prepared and ready to experience the joy of producing your very own nitric acid!
Edit: - options for suck back
When distilling without vacuum I use a dry dreschel flask between the receiver and scrubber, but set up in the opposite direction to the scrubber if
that makes sense, inlet has the short tube outlet has the long one.
[Edited on 24-3-2021 by B(a)P] |
Thanks for the suggestions and I had forgotten about suck-back in this type of distillation. I've only had this happen when trying to make stronger
ammonia solution (stronger than 30%). Eveything was going great until suddenly I had 800ml of the new ammonia solution rocket back through the
condenser and into the boiling flask. I had used suck-back protection but I forget what happened that made it not work right. The next time I was
making ammonia again, but had finished and I was allowing it to cool down, and didn't know/remember I had to remove the receiver flask as soon as the
temp was turned off on the boiling flask. I was in the other room and I heard this noise that sounded like a jet (pulling back almost 2L of liquid)
and I gave up after this.
I have distilled alcholos from "mash" many times, LOTS of 1L flasks heated (using 10-13% ABV) and I did this all with only vinyl tubing connecting the
boiling flask & the receiver. I ended up with almost 40 gallons of 75-80% clear rum, so IDK even know how many flasks I filled to boil, but I had
it running next to my desk in the winter, so I turned my heat off and allowed this to heat my room(s)!
AS far as suck back I'm a little confused. I have about 200-300 pieces of glassware which I do not know their purpose (they all came lots with much
more inventory). The issue I'm taking with suck back protection, I'm not sure how this is possible with a sealed container.
Le's say I use a 1 or 2L Erlenmeyer flask (with 24/40 ground neck joint). I can get 2 Pyrex tubes down there no problem, but getting a funnel through
the neck opening seems impossible & when I did this before, I always used beakers with the largest funnel the would sit inside it. Maybe I'm
missing the point here or just don't know what you are talking about.
This is the method I was talking about. I can't figure out what kind of vessel to use to allow a funnel inside but also be able to seal the lid so I
can draw vacuum across the daisy chained pump, containers, receiving flask, condenser & boiling flask. If you could give me some ideas, I'd be
extremely grateful.
As far as the nitrates, I have KNO3 (stump remover), which wasn't nearly as pure as I hoped and I was shorted almost 240 grams out of 2 1lb bottles!
One bottle was 310g including container and spout, the other was about 360g! Both are supposed to be 454 to 475g + weight of bottle. I purified 3x
by boiling in distilled water, slowly cooling and removing 8%, 5% & 3% of the last part of the liquid that hadn't recrystallized yet. It also had
a very strong garlicky smell, I've never noticed this before, and I have to say , it looks very much like garlic powder...
So if you have any suggestions on containers for this, I'm all ears. I guess I could use some wide-mouth glass jars (like 5-8x thicker than good
quality beakers) but they aren't borosilicate. I just don't know how I could fit the funnel into a beaker, put a lit on and seal it, unless there was
another method than what you explained
I've added some pretty bad drawings below to show what I'm picturing of the suck back protection and why I can't see how it would work. I really
apologize for the quality of these images but my 4 graphics programs are all crashing for some reason - it's odd that 4 diff programs (and both my
browsers are crashing), so I had to use the most basic program with Windows paint.
So can't someone explain how to do suck back on a step like this that might work?
https://www.youtube.com/watch?v=kqQSpRus-t0
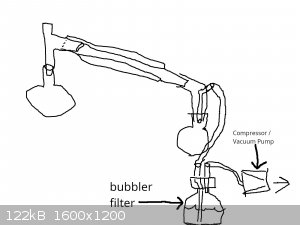 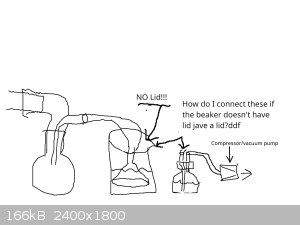
|
|
|
Fyndium
International Hazard
    
Posts: 1192
Registered: 12-7-2020
Location: Not in USA
Member Is Offline
|
|
You can use check valve to prevent suckback. My aspirator has an in-built valve, and I found it out only after I heard that simple aspirators don't
have one and wondered why mine had never had a suckback.
You can enclose beakers and other stuff in ziploc bags and seal them against any tubes or pipes going in and out. Or then, if it's an option, get a
long and cheap garden hose or other tube that extents outdoors and vent the gases there. Or if you have ventilation outlet and know how the system
works, let the gases there.
|
|
|
RogueRose
International Hazard
    
Posts: 1593
Registered: 16-6-2014
Member Is Offline
|
|
Quote: Originally posted by Fyndium  | You can use check valve to prevent suckback. My aspirator has an in-built valve, and I found it out only after I heard that simple aspirators don't
have one and wondered why mine had never had a suckback.
You can enclose beakers and other stuff in ziploc bags and seal them against any tubes or pipes going in and out. Or then, if it's an option, get a
long and cheap garden hose or other tube that extents outdoors and vent the gases there. Or if you have ventilation outlet and know how the system
works, let the gases there. |
Hmm, I've never heard of being able to use suck back protection in a beaker while also using it as vacuum. I'm trying figure out a way to do this.
I do have an idea to use this stuff that is inside/outside weather stripping & caulking cord. The stuff is really awesome and can usually be
found in either the windows/doors section or plumbing section. I think I got 90ft x 1" wide (8 cords each .125" wide). This stuff is really amazing
and I've found more uses for it than I can count. It's similar to that poster tac putty that you can use to stick stuff to the wall w/o putting holes
in the wall (and at $6/7 for 2 oz, vs $6/7 for the weatherstrip/caulk but you get 10x as much and it sticks MUCH MUCH better).
I've used this strip to seal all kinds of misc glassware that was mis-matched in size (by up to 1/3" difference). I think I can place a plastic lid
on a beaker with holes for the tubes & suckback funnel.
I'll see if I have any pics of me using this, you'd e amazed at the things I rigged up when I didn't have any proper glassware and it always held up
to temps up to 300+ and down to well below freezing, probably -20 or even lower. It also even does well wet and if solvents get on it - it seems
kind of impervious to everything I've thrown at it.
Here's a link to the manufacturer site, it doesn't go into very deep detail, but for $6, it's well worth it to have it on hand to plug hole or even
fix some cracked glass (it's not ment to be permenant like JB weld or anything). It stays softish and I've reused it a number or times
https://www.frostking.com/products/weatherstripping/mortite-...
Here's the 90ft product at WalMart
https://www.walmart.com/ip/Frost-King-Indoor-Outdoor-B2-Mort...
If you have the choice of the 9.5oz or the 19oz for a very small $$ difference, I'd HIGHLY suggest the larger one as min's been sitting exposed on a
shelf for 14 years and it works just as well as when it was new, so it lasts a LONG time and you'll fine many little uses for it. Get the bigger one
if they have it!
Just a quick question about the shape of the funnel, IDK if it matters that this one is pretty deep as I've seen a bunch that have much more sloped
sides up to the rim. Do you think this will work b/c the diameter is great fr what I want and it probably has actually 2x the volume of most funnel's
I've used in the past that were relatively the same size. BTW, that's a standard 12oz can next to it.

[Edited on 3-26-2021 by RogueRose]
|
|
|
RustyShackleford
Hazard to Others
  
Posts: 200
Registered: 10-12-2020
Location: Northern Europe
Member Is Offline
|
|
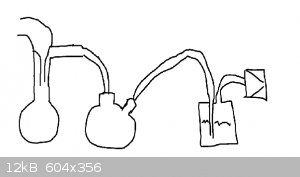
|
|
|
B(a)P
International Hazard
    
Posts: 1139
Registered: 29-9-2019
Member Is Offline
Mood: Festive
|
|
Quote: Originally posted by RogueRose  |
Just a quick question about the shape of the funnel, IDK if it matters that this one is pretty deep as I've seen a bunch that have much more sloped
sides up to the rim. Do you think this will work b/c the diameter is great fr what I want and it probably has actually 2x the volume of most funnel's
I've used in the past that were relatively the same size. BTW, that's a standard 12oz can next to it.
|
Deeper the better because it gives you more freeboard.
The idea is to have enough water to catch the NO2 / acid vapour, but not so much that if all water is sucked in the funnel it is able to
enter the connected tubing.
Edit - this set up will not work with vacuum distillation.
If you are sticking with vacuum filtration make another of your bubble filters and put it in dry and backwards (or just with two short lines) compared
to the other. Place the dry bubble filter between the receiver and the bubble filter with water.
[Edited on 26-3-2021 by B(a)P]
|
|
|
Fyndium
International Hazard
    
Posts: 1192
Registered: 12-7-2020
Location: Not in USA
Member Is Offline
|
|
Of course you cannot use inverted funnel with vacuum, unless you have some very special equipment, like flanged reactors, etc. Any beaker would likely
implode upon vacuum, if you were able to seal it. I thought this was for ntp operations.
When I get past certain point of complexity, I return to baseline and rethink the concept. Otherwise you will find that you are attempting to make
exceedingly complex and likely expensive system that might not work.
For simplicity, use setup as B(a)P described. If you don't have dry ice and acetone, you can make a batch of calcium chloride solution and freeze it
as low as you can get (freezer, etc). I just vacuum stripped water off a hygroscopic liquid, and I used first ordinary liebig to condense everything,
and then I ran it through a graham condenser - both cooled with salt ice water, and finally the receiver flask was immersed in salt ice water. The
coldfinger was immersed in CaCl2 bath at -30C. Very little water was found frozen in the coldfinger, so the apparatus apparently worked pretty well.
|
|
|
zed
International Hazard
    
Posts: 2283
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
https://www.youtube.com/watch?v=tYLlkTDstmo
Personally, I don't like the idea of circulating NaOH solutions through an aspirator to scrub noxious gases.
I'd rather bubble the gases through a scrubbing solution. Assisted by vacuum.
You need a vacuum trap between your product, and your scrubbing solution. You might need another vacuum trap between your scrubbing solution and your
vacuum source.
I like an aspirator pump... outside somewhere. Other folks do it differently. Just depends.
I have always managed to avert disaster. Sometimes, just barely.
If you are generating lots of toxic gas, an inverted funnel, probably won't be adequate.
|
|
|
Fyndium
International Hazard
    
Posts: 1192
Registered: 12-7-2020
Location: Not in USA
Member Is Offline
|
|
A lot of toxic gases require venting it directly outdoors either via duct ventilation or through a long tube. I've got experience using both.
Using NaOH solution with aspirator and pump that doesn't get damaged and using check valve between your apparatus and pump I don't see a problem here.
The solution mustn't be 50%, but more like 5%. Perhaps an issue may raise with cooling, as the circulating water gets heated, it will lose efficiency
and you need some closed circuit coolant system to cool it in order not to dilute it.
|
|
|
RogueRose
International Hazard
    
Posts: 1593
Registered: 16-6-2014
Member Is Offline
|
|
Thanks for all the great replies! I'm definetely going to be buying an aspirator and upgrading the motor on my "100 PSI) pump if it isn't sufficient
to pull good vacuum. But I also think that it would be really beneficial to add some check valves in between the receiving flask, empty flask &
scrubbing flask (so I need 2 I guess).
I also have a number of compressors that I use when I need to do either high flow or high pressure by running them in series or parallel. Some of
these also are used at times to draw vacuum when I need to evacuate something very quickly (like vacuum forming). So I'll be ordering some valves for
this as well.
But my biggest question is if I'm using these inline with the receiving flask -> empty -> scrubber -> dryer (maybe) -> vacuum there is a
chance that 2 of these may end up getting suck back with liquids but I can't find info if they work with both liquid and gas/air. I'm thinking I'll
take a risk and see if they handle liquid & if not, then use them for something else.
Finally, IDK what size I should be looking to get. Most of my barbs accept 3/8" vinyl tubing but these can often collapse under vacuum so I've used
reducers to 3/16 or 1/8" but I've been told that using smaller tubes slows down the vacuum process by A LOT! This is why those $10-30K vacuum pumps
have a 2-3" connection port with steel reinforced/ribbed hose. But I'm not sure if it's going to matter for the volume & quantities I'll be
working with as to what size hose I use.
Edit - I forgot to add links to check valves I'm looking at. They are priced extremely well if anyone is interested.
Check valves
https://www.usplastic.com/catalog/default.aspx?catid=1361&am...
I think these valves are the best option for the price
https://www.usplastic.com/catalog/item.aspx?itemid=23369
I’ thinking of this unit:
https://www.usplastic.com/catalog/item.aspx?itemid=36844
Hose barb – Kaynar or PolyProp & Viton diaphram
Kynar = PVDF
Viton = “Flourine rubber”
Check valves – Polyprop or Kynar
https://www.usplastic.com/catalog/item.aspx?itemid=121210&am...
Hose barb – Kaynar or PolyProp & Viton diaphram
https://www.usplastic.com/catalog/item.aspx?itemid=137121
8mm to 10mm Kartell Polypropylene Check Valves
https://www.usplastic.com/catalog/item.aspx?itemid=119286&am...
Hose Barb Black Kynar® Check Valve with 1 psi Spring & Viton™ Seal
https://www.usplastic.com/catalog/item.aspx?itemid=24234&...
Hose Barb Black Kynar® Mini Check Valve with Viton™ Seals
[Edited on 3-31-2021 by RogueRose]
|
|
|
Belowzero
Hazard to Others
  
Posts: 173
Registered: 6-5-2020
Location: Member Is Offline
Member Is Offline
|
|
What products would be formed when NO2 is passed through a solution of KOH.
Would it form KNO2 or KNO3 or both ?
I can also imagine some of it would decompose to N2 and O2.
Not quite sure how to consider this reaction.
Would a sodiumbicarbonate solution be as effective and perhaps more economical?
[Edited on 31-3-2021 by Belowzero]
|
|
|
zed
International Hazard
    
Posts: 2283
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
It would be safer. The aspirator ejects water (mixed with air or gases), under pressure. I have misgivings about using a device that does that,
while the water is charged with KOH or NaOH.
Accidents happen, and I would like to continue, to use my eyes for seeing.
Bicarbonate is pretty basic, but I am assuming it is less dangerous than KOH or NaOH.
|
|
|
RogueRose
International Hazard
    
Posts: 1593
Registered: 16-6-2014
Member Is Offline
|
|
Quote: Originally posted by zed  | It would be safer. The aspirator ejects water (mixed with air or gases), under pressure. I have misgivings about using a device that does that,
while the water is charged with KOH or NaOH.
Accidents happen, and I would like to continue, to use my eyes for seeing.
Bicarbonate is pretty basic, but I am assuming it is less dangerous than KOH or NaOH. |
Do you think NaCO3 would be better than bicarb as bicarb does not have a high solubility?
Also, if using an aspirator pump, wouldn't the water just absorb any of the NO2 gas that comes over, or maybe even adding a slight amount of H2O2? I
was planning on chilling the aspirator fluid as it seems that it should absorb NO2 faster and it should draw a better vacuum.
My circulation process will be in a closed container so I'm not worried about any of the water/solution splashing out.
|
|
|
arkoma
Redneck Overlord
      
Posts: 1761
Registered: 3-2-2014
Location: On a Big Blue Marble hurtling through space
Member Is Offline
Mood: украї́нська
|
|
Been sitting up all night; on my fifth 100gm (KNO3) batch of Nitric. Been using this scrubberb all night. from the RIGHT is gas take off adapter on
250 2 neck rbf >>> empty wash bottle >>>> wash bottle with NaOH solution, attached to vac pump JUST IN CASE. Rather fry my
rotary vane than lungs. Not one misbehaving whiff of NO2.

"We believe the knowledge and cultural heritage of mankind should be accessible to all people around the world, regardless of their wealth, social
status, nationality, citizenship, etc" z-lib
|
|
|
Fyndium
International Hazard
    
Posts: 1192
Registered: 12-7-2020
Location: Not in USA
Member Is Offline
|
|
I was thinking using an aspirator with activated water medium to scrub volatile fumes. What idea I came up with is venting the reaction apparatus into
a vessel that has a controlled leak, and from there into an aspirator pump, that circulates a larger body mass. The activating agent can be either
basic or acidic or of other nature to form a stable, water soluble compound.
The leaking vessel, that can essentially be a 3-necked flask with reflux condenser and an outlet valve that is at least partially opened, and second
outlet from reaction vessel that the gases are led into, and another outlet into aspirator. The flask can be also filled halfway with solution that
has a neutralizing agent dissolved, so it will already scrub part of the volatiles.
For example, venting a reaction that generates nitric oxide. NO is not water soluble, but it will react with oxygen tor form NO2, which is, and this
an be easily neutralized with a basic element like NaOH or Na2CO3.
A reaction that vents basic gases like ammonia, will have the medium changed from basic to acidic, for example citric acid, or other similar acid that
is suitable for the process but is not too strong to corrode the pump.
The leak valve allows to equalize pressure and allow for proper flow direction, and it also acts as a release valve in case such pressure deviation
occurs that overruns the aspirator for any reason.
The aspirator would not have to be ran at full rate, but it basically needs to absorb any reaction volatiles.
Finally, the system can be installed inside a fume hood that is extracted with activated carbon filter, so most residues would be filtered.
|
|
|
macckone
Dispenser of practical lab wisdom
    
Posts: 2168
Registered: 1-3-2013
Location: Over a mile high
Member Is Offline
Mood: Electrical
|
|
Hints for NO2 absorbion:
It is a liquid below 21C.
Cold hydrogen peroxide will rapidly convert it to nitric acid as will ozone.
I use a couple of plastic coke bottles with clear pvc tubing for a trap.
One functions as the suck back prevention and the other as the scrubber.
short tube on in and out of the pair and long tube connecting the pair.
Cheap and easy.
Weldwood Contact Cement is chlorinated rubber in a toluene solution, it is very resistant to nitric oxides and nitric acid.
At least no leak with the same set up for years, barring my recent glass reactor vessel exploding, which was bound to happen.
|
|
|
Fyndium
International Hazard
    
Posts: 1192
Registered: 12-7-2020
Location: Not in USA
Member Is Offline
|
|
The concept of using two bottles as a suckback is intriguing. Pressure flucutations causes the liquid to travel between the containers, creating a
little backpressure, but it will also increase the absorption rate.
Issue with me is a reaction generates a lot of nitric oxide, which first needs to convert to NO2 to be able to be absorbed.
https://doi.org/10.1002/ep.10075
It appears that using hypochlorite as a medium would oxidize and absorb most of the NOx.
|
|
|
macckone
Dispenser of practical lab wisdom
    
Posts: 2168
Registered: 1-3-2013
Location: Over a mile high
Member Is Offline
Mood: Electrical
|
|
NO is significantly less dangerous than NO2.
If the purpose is more HNO3, then that is important.
If it is to reduce danger then less but not zero concern.
[Edited on 4-9-2021 by macckone]
|
|
|