werdy666
Harmless

Posts: 9
Registered: 19-1-2021
Location: NSW, Australia
Member Is Offline
Mood: Chilled
|
|
Copper II Chloride From Cu2(OH)2CO3 & HCl
Hi 
I saw a couple of you tube videos about making Copper II Chloride from Basic Copper Carbonate and Hydrochloric Acid.
This one in particular..
https://www.youtube.com/watch?v=iychy8RJq0M&t=24s
He puts 5grams of Copper carbonate with 15ml 36% HCl + H2O up to 40ml
I have also been visiting this site
https://en.intl.chemicalaid.com/tools/reactionstoichiometry....
I've been using this to do the Stoichiometry for me(Cause I am lazy!) lol
However when I try to use an Acid, Such as HCl, I have no clue how to interpret grams to the volume of acid. The main issue is that my HCl is not 36%.
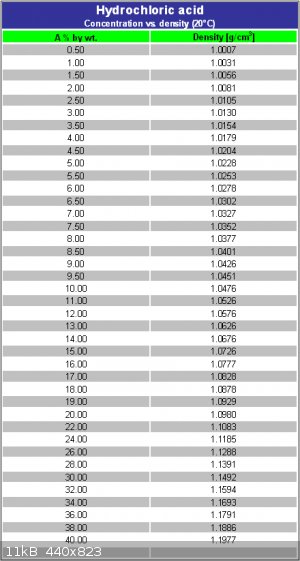
My HCl is 320g/1000ml. From some tables I have looked up that indicates to me I have a concentration of 27% or there abouts.
How do I use the above Stoichiometry Calculator for liquids like Acids with different Concentrations in my calculations?
How does the guy in the video know to put 15ml of 36% HCl with 5g Copper Carbonate. (What calculations did he do and how did he do it?  ) )
Any tips or sites that can help me understand how it is calculated would be great for my learning.
My maths tells me that based on 15ml of 36% HCl with 5g Copper Carbonate, My HCl at 27% I need 20ml for 5g Copper Carbonate.
I hope that made sense.... lol 
|
|
|
outer_limits
Hazard to Others
  
Posts: 139
Registered: 3-3-2020
Member Is Offline
Mood: hybridized
|
|
You don't use any stoichiometry calculator.
Instead, you learn about concentration, moles and calculations. Then you can calculate everything you need.
|
|
|
artemov
Hazard to Others
  
Posts: 181
Registered: 22-8-2018
Member Is Offline
|
|
Quote: Originally posted by werdy666  | Hi 
My maths tells me that based on 15ml of 36% HCl with 5g Copper Carbonate, My HCl at 27% I need 20ml for 5g Copper Carbonate.
|
You need to factor in the density difference between 36% hcl and 27% hcl as well. So the equivalent amount is about 20.8ml.
Also after some calculation, the amount of hcl he used is almost a 2x excess.
[Edited on 20-1-2021 by artemov]
|
|
|
woelen
Super Administrator
        
Posts: 8012
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
A practical method: Just use some excess copper carbonate and allow the mix to react for a day or so. This will consume all acid and the remaining
copper carbonate settles at the bottom and can easily be separated from the liquid.
A method, giving more knowledge: Study some books on stoichiometry of reactions and how to compute weights from molar ratios. If you show that you put
some effort in this and come up with your own solutions to such problems, then there will always be people here (including me) who are willing to help
you with certain things or to clarify any errors if they are present.
|
|
|
werdy666
Harmless

Posts: 9
Registered: 19-1-2021
Location: NSW, Australia
Member Is Offline
Mood: Chilled
|
|
woelen Typed.....
| Quote: |
A practical method: Just use some excess copper carbonate and allow the mix to react for a day or so. This will consume all acid and the remaining
copper carbonate settles at the bottom and can easily be separated from the liquid. |
The only issue is that as artemov stated that the video shows almost 2x excess of HCL. Without knowing how to do the calculations I could be there all
night adding Copper carbonate to the solution and never learning  (well maybe not
all night (well maybe not
all night  ) )
I will see if I can find a beginner online textbook to help me learn and understand concentrations, moles, calculations, density and molar ratios.
|
|
|
artemov
Hazard to Others
  
Posts: 181
Registered: 22-8-2018
Member Is Offline
|
|
Quote: Originally posted by werdy666  |
The only issue is that as artemov stated that the video shows almost 2x excess of HCL. Without knowing how to do the calculations I could be there all
night adding Copper carbonate to the solution and never learning  (well maybe not
all night (well maybe not
all night  ) )
I will see if I can find a beginner online textbook to help me learn and understand concentrations, moles, calculations, density and molar ratios.
|
This is for my synthesis of nickel(II) chloride. This is how i normally do my calculations (this is definitely not the best or only way).
Note that I used mass instead of volume even for liquids, as it is more accurate for me to measure mass and I dun have to care about the density of
the liquids.
1. The first thing you need to do is write down the reaction equation,
2. then decide on the amt to use for one of the reactant,
3. calculate the molar equivalent,
4. use the equation to find the molar equivalent for the rest of the reactants,
5. then work backwards to calculate the required amts.
You can also calculate the theoretical yield easily.
Follow the steps and see if you can do it. The two step 5s may be a bit difficult.

[Edited on 20-1-2021 by artemov]
|
|
|
outer_limits
Hazard to Others
  
Posts: 139
Registered: 3-3-2020
Member Is Offline
Mood: hybridized
|
|
Quote: Originally posted by werdy666  |
I will see if I can find a beginner online textbook to help me learn and understand concentrations, moles, calculations, density and molar ratios.
|
https://chem.libretexts.org/Bookshelves/General_Chemistry/Ma...
[Edited on 20-1-2021 by outer_limits]
|
|
|