| Pages:
1
2 |
Diachrynic
Hazard to Others
  
Posts: 226
Registered: 23-9-2017
Location: western spiral arm of the galaxy
Member Is Offline
Mood: zenosyne
|
|
Purple acid, N2O2HSO4
woelen will probably like this one ^^
I think I found this one on atomistry, which seems to be down at the moment. The relevant page, thankfully, is saved here in the Wayback Machine.
For completeness sake, I will include the full quote right here:
| Quote: | Sulphonitronic Acid
Sulphonitronic Acid or "Purple Acid"-For a considerable time the existence of an unstable compound which yields a purple solution in sulphuric acid
has been known. Of various names which have been suggested for this compound, the designation "sulphonitronic acid" possesses the advantage of
indicating the nature of the chief constituent elements without committing itself to any definite conception of the structure or constitution. The
acid has been regarded as a derivative of quadrivalent nitrogen, viz. " nitrosisulphonic acid," O:N(OH).SO2.OH, or peroxylaminic acid,
NO(SO2.OH)2. Unfortunately the question of the actual constitution is still undecided, and even the composition is uncertain.
According to more recent investigations it appears probable that the " acid" is either an oxide of nitrogen intermediate between NO and
N2O3, or a compound of sulphuric acid with such an oxide. On account of this uncertainty the compound is frequently referred to
merely as "purple acid" (also "blue acid " and "violet acid"). It has possibly acquired undue importance on account of its occurrence as an
intermediate product in the "lead chamber" process for the manufacture of sulphuric acid.
Formation:
The coloured solution in sulphuric acid is obtainable by passing nitrogen dioxide with air into a saturated solution of sulphur dioxide in diluted
sulphuric acid (1:1 by volume) at 0° C. It can also be formed by the addition of sodium hydrogen sulphite to a solution of nitrosulphonic
(nitrosylsulphuric) acid, produced by dissolving sodium nitrite in slightly diluted sulphuric acid. These methods depend on the reduction of the
nitrosulphonic acid by sulphurous acid or sulphur dioxide; the reduction can also be effected by metals, e.g. mercury.
Properties:
The blue solution is unstable and decomposes slowly, with formation of sulphuric acid, sulphur dioxide and nitrogen dioxide. When shaken with air or
submitted to oxidation by chlorine, nitric acid or hydrogen peroxide, conversion into nitrosulphonic acid is effected, brown fumes being liberated.
Dilution with water also destroys the coloured substance. If strongly cooled, the solution changes to an intense red, so that if a solution is too
weak to possess a marked colour at the ordinary temperature, the presence of the "purple acid" can easily be detected by cooling in a mixture of
acetone and solid carbon dioxide.
Certain of the salts in solution have a stronger colour than the acid, and in some cases are more stable; thus, a deep blue solution of the copper
salt may be obtained by the reduction of nitrosulphonic acid (in sulphuric acid) by mercury in the presence of copper. A suggestion has been made that
the colour in the "brown ring" test for a nitrate is due to the formation of the ferrous salt of "purple acid," but this is improbable.
|
Atomistry, as so often, is very interesting, but gets some things wrong.
A better source of information is of course the Gmelin Handbook of inorganic chemistry series, which sadly is non digitalized. The information is from
Sulfur part B pages 1630ff.

They call this compound Nitrogen Oxide Nitrosyl Hydrogen Sulfate with the formula N2O2HSO4, or better
(ON.NO)HSO4, the most common name is "blue acid" but "purple acid" describes the color much better.
This compound can be formed in a variety of ways, but it is unstable, can't be isolated (probably) and even in conc. sulfuric acid only stable for a
few days. Nonetheless, it is very impressive.
It can either form from the reduction of NOHSO4 in sulfuric acid by metals, sulfur dioxide, some organic compounds, and nitrogen monooxide
under pressure. There are some more but more exotic methods.
Some more information can be found here: Seel, Ficke, Riel, Völkl, 1953. The doi is 10.1515/znb-1953-1011
So far, I have made the compound in three different ways, and I tend to explore at a later time what the optimal conditions for this are.
The first method I did was this:
Some 95-97% sulfuric acid was taken and some small amount of NaNO2 was added to it. This was shaken, but not everything dissolves. Then
some 85% formic acid is dripped into it. THIS PRODUCES CARBON MONOXIDE! The liquid foams a bit from the evolved CO. At this point I
was already expecting a purple color, but nothing happend. So I put everything into a hot water bath for a bit to get rid of at least some CO. I also
read that copper is a catalyst for this, so I added a blank piece of copper wire. And within a few seconds, vibrantly purple streaks started to form.
After shaking the entire solution was dark purple.


I left the solution standing like this for three or four days, and the color slowly faded and finally was colorless again.
In retrospect, the formic acid probably didn't do much apart from diluting the sulfuric acid, but I read it should have been able to reduce the
NOHSO4 by itself. It seems it doesn't. I assume the formic acid can be left out, but I haven't tried that yet.
Someone might say this is a copper complex, but there is good reason to believe that this is not true (although atomistry mentions something of a
copper salt(?) of this which has an even stronger color, I guess it could be it?), because the same color appears with zinc:
To some 95-97% sulfuric acid some NaNO2 is added, shaken until mostly dissolved, then some pure zinc powder is added. This takes a much
longer time than before. Eventually however, the solution turns purple.

I added more zinc and the solution eventually had a quite dark color, compareable to the one in the first experiment.
Yet another experiment can be performed, and this time I made sure to record masses:
Everything was around 0 °C cold. I added 1.88 g (about 1 mL) of the 95-97% H2SO4 to a test tube, then I added 70 mg of
NaNO2 to it, stirred it around with a glass rod and left it there for ten minutes, didn't all dissolve, very slow gas evolution. Then I
added 40 mg of Na2S2O5 to it and stirred again, not much gas evolution, and no full dissolution either, left again
for about 10 minutes. Then I put it into a 30 °C water bath where some gas evolved, but not much, the solution started clearing up and after ten
minutes of standing outside the water bath it was mostly clear. Then I started adding distilled water dropwise to the test tube so it ran down the
sides. On hitting the acid some gas evolved, and the acid started turning purple. I think I added about 5-6 drops, and shook it a bit, then left to
stand for a few minutes for the bubbles that formed to rise.

The layering occurs because mixing in the test tube is fairly bad and the water only entered the top portion so far. The color continues to darken.
Too much water will likely (according to what I read) destroy the compound again. Indeed: When the solution was diluted with an equal volume of water,
all purple color faded away and some brown gas was evolved and it smelled of NO2.
I'm fairly confident in saying I have successfully produced a solution of N2O2HSO4 in sulfuric acid.
I hope that I can add the relevant Gmelin pages to this thread at some point. Information seems to be sparse about it.
[Edited on 5-1-2021 by Diachrynic]
we apologize for the inconvenience
|
|
|
RustyShackleford
Hazard to Others
  
Posts: 201
Registered: 10-12-2020
Location: Northern Europe
Member Is Offline
|
|
Good writeup! i recall trying it on your behalf some months ago but never adding the copper wire, so i was dissapointed.
|
|
|
Bedlasky
International Hazard
    
Posts: 1251
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
Very nice! I will definetely try it! I plan to make some highly electrophilic species in conc. H2SO4 and oleum, so this will be nice addition to these
experiments.
|
|
|
woelen
Super Administrator
        
Posts: 8080
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
This really is interesting! Thanks for sharing this. I certainly will try this myself and maybe I'll add a webpage to my website on this subject if I
succeed. I never heard about this compound. Chemistry keeps surprising me 
|
|
|
EthidiumBromide
Harmless

Posts: 42
Registered: 27-9-2020
Member Is Offline
Mood: Effervescent
|
|
This is very interesting, I have to try this when I have some spare time. Thanks for sharing!
|
|
|
Diachrynic
Hazard to Others
  
Posts: 226
Registered: 23-9-2017
Location: western spiral arm of the galaxy
Member Is Offline
Mood: zenosyne
|
|
I'm glad that I am not the only one that finds this fascinating ^^
RustyShackleford, I remember that. It seems that the original claim that formic acid reduces it by itself isn't true or as easy to
reproduce. Either way, I think with the CO involved it is an impractical method when better alternatives exist.
Bedlasky, that sounds really nice, I hope the experiments work!
woelen, please do! That would be amazing to see since I'm a big fan of your website.
EthidiumBromide, thank you and good luck! I hope it works.
Here are some papers papers on the subject (all of which are in German):
Manchot, 1910,, "On the alleged nitrosisulfonic acid by Raschig (Sabatiers nitrosodisulfonic acid) and the theory of the lead chamber process",
the doi is 10.1002/ange.19100234501
Attachment: Manchot1910.pdf (222kB)
This file has been downloaded 399 times
Manchot, 1911, "About the 'blue acid' (Raschigs nitrosisulfonic acid), reply to Mr. Raschig", the doi is 10.1002/ange.19110240106
Attachment: Manchot1911.pdf (350kB)
This file has been downloaded 418 times
Manchot, 1912, "On the recognition of the 'blue acid' (the reduction product of nitrosylsulfuric acid)", the doi is 10.1002/ange.19120252104
Attachment: Manchot1912.pdf (453kB)
This file has been downloaded 407 times
Seel, Ficke, Riel, Völkl, 1953,, "Notes about nitrogen oxide - nitrosyl salts", the doi is 10.1515/znb-1953-1011
Attachment: Seel1953.pdf (2MB)
This file has been downloaded 406 times
Wolf, Hummel, 1967, "On the reduction of nitrosylsulfuric acid in conc. sulfuric acid", the doi is 10.1002/zaac.19673540103
Attachment: Wolf1967.pdf (116kB)
This file has been downloaded 411 times
[Edit: The file extensions of the pdfs were missing]
[Edited on 6-1-2021 by Diachrynic]
we apologize for the inconvenience
|
|
|
Diachrynic
Hazard to Others
  
Posts: 226
Registered: 23-9-2017
Location: western spiral arm of the galaxy
Member Is Offline
Mood: zenosyne
|
|
I conducted abother experiment to see if there was indeed a "more strongly colored copper salt" as atomistry claimed.
I mixed 1.90 g (about 1 mL) of 95-97% sulfuric acid and 70 mg of NaNO2, put it into a water bath of 40-50 °C until it all dissolved (which
it did quickly with just minor gas evolution), took it out and let it cool back down somewhat, then I added 40 mg of
Na2S2O5 into it (this time, as the solution wasn't as strongly cooled as in the last experiment, some foaming occured
but it wasn't a problem). The solution was put for a minute or so back in the water bath and stirred, again, an almost colorless solution was
obtained.
Now a solution of 50 mg CuSO4 · 5 H2O in 1 g of water was prepared which amounts to about 0.2 mol/L. I added 5 drops to the
acid which was accompanied with some gas evolution. Even with the first drop a darker purple color formed than without copper(II). Again, bad mixing
in the test tube caused a layering of a colored upper phase, despite everything being fully miscible.
So it seems the dark purple color in the experiment with the formic acid could be the copper salt atomistry mentions. Since a similar, although less
intense, color is obtained without copper as well the existence of N2O2HSO4 is not contradicted by this.



we apologize for the inconvenience
|
|
|
Boffis
International Hazard
    
Posts: 1897
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
What an interesting find! Thank for the attached papers too, some light bedtime reading 
|
|
|
woelen
Super Administrator
        
Posts: 8080
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Several years ago, I discovered a very intensely colored blue complex of copper with nitrite in strongly acidic solution. I have written a webpage
about that, 10 years ago or so.
Your experiment provides new light on this. In my webpage I write that chloride is necessary for formation of the complex, but maybe any reductor will
do.
https://woelen.homescience.net/science/chem/riddles/copper+n...
This splits the situation into two different directions. One direction is the situation with the presence of copper, the other is the situation
without any copper. The latter one is most striking and I really want to see this myself.
With zinc as reductor, you also get a faint purple color, but how sure are you that the zinc is absolutely free of copper? Given the intense color of
the copper complex, even a tiny amount like 0.01% of copper in the zinc could give rise to the color.
We should try to get the purple compound with a reductor, which certainly will not contain any copper, e.g. Na2S2O5 or gaseous SO2. I have a bottle
with a solution of 5 to 6% SO2 (I once made this for experiments with V2O5, trying to convert that to VOSO4 without any other cations in the system),
and I could try experimenting with this (e.g. diluting 3 volumes of reagent grade H2SO4 with 1 volume of the solution of SO2) and adding NaNO2 to
that. Metallic reductors like zinc, aluminium or magnesium I do not really trust. Many commercially available metals may contain traces of copper.
|
|
|
Diachrynic
Hazard to Others
  
Posts: 226
Registered: 23-9-2017
Location: western spiral arm of the galaxy
Member Is Offline
Mood: zenosyne
|
|
Boffis, thank you very much! When I am able to include the Gmelin pages (and maybe even translate them to English) here there will be
a bunch more reading material too!
This is very interesting! There is one very remarkable difference between the experiments however. In your experiment, you used 20% sulfuric acid.
This complex however is unstable at such concentrations. To demonstrate this, the following experiment can be performed:
To half of the blue solution prepared earlier, about 0.5 mL, was added water dropwise. About 7 drops of water were added, which the scale indicated as
a gain in weight of 160 mg. The solution evolved brown NO2 gas and warmed, as expected from sulfuric acid. The purple color disappeared
within seconds completely.

An estimation of the concentration: 7 drops weighed about 160 mg here, plus 5 drops earlier divided by two means about 200 mg of water were added to
about 0.95 g of 95-97% sulfuric acid, which means a concentration of about 75% is remaining in the colorless test tube.
It is however likely that the deep blue coloration is due to a complex between the N2O2+ and the Cu2+
(what atomistry calls a "copper salt" of the purple acid), since according to your experiments it can complex with NO/NO2 and related
species. Given that chloride seems to be required, it could also involve nitrosyl chloride.
EDIT: In fact, I have found mention of this in Sharp, Thorley, 1963, "670. The infrared spectrum of the nitrosonium ion", the doi is 10.1039/JR9630003557
Weirdly enough they seem to be talking about blue Cu+ complexes. Strange.
Attachment: Sharp1963.pdf (367kB)
This file has been downloaded 449 times

Quote: Originally posted by woelen  | This splits the situation into two different directions. One direction is the situation with the presence of copper, the other is the situation
without any copper. The latter one is most striking and I really want to see this myself.
With zinc as reductor, you also get a faint purple color, but how sure are you that the zinc is absolutely free of copper? Given the intense color of
the copper complex, even a tiny amount like 0.01% of copper in the zinc could give rise to the color. |
This is a valid concern, even if my zinc is labled as 99.995% pure. There could be copper as an impurity and I agree that the experiment is more
impressive without any metals at all. Gaseous sulfur dioxide should be a way to make it absolutely metal free. I despise the smell of it however and
adding metabisulfite is a lot more convenient, but sulfur dioxide does work as well, according to the literature.
[Edited on 6-1-2021 by Diachrynic]
[Edited on 6-1-2021 by Diachrynic]
we apologize for the inconvenience
|
|
|
Diachrynic
Hazard to Others
  
Posts: 226
Registered: 23-9-2017
Location: western spiral arm of the galaxy
Member Is Offline
Mood: zenosyne
|
|
Here are the relevant Gmelin pages. Might translate them to english later if I have the time.
Attachment: N2O2HSO4.pdf (1.5MB)
This file has been downloaded 438 times
we apologize for the inconvenience
|
|
|
Bedlasky
International Hazard
    
Posts: 1251
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
Look at this:
https://europepmc.org/articles/pmc2908284/bin/nihms220726-su...
Speaking of copper nitrite complexes, this one is quite interesting:
https://www.sciencemadness.org/whisper/viewthread.php?tid=15...
|
|
|
woelen
Super Administrator
        
Posts: 8080
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Very interesting read. Apparently it is important to have some water in the mix (e.g. 20%) to obtain the purple compound. I have a little doubt about
the NO + NO(+) situation, described in the german paper. In that paper it is written that passing NO over a solution of NO(+)/HSO4(-) in 80% H2SO4
forms the purple ion [ON.NO](+). But why have I never observed this when dissolving NaNO2 in sulfuric acid of varying concentrations? Simply adding
NaNO2 to e.g. 80% H2SO4 should give at least some hints of blue/purple. There always is some decomposition of nitrite with formation of NO and HNO3,
no matter how caerfully you add the nitrite to the acid.
Time to take out the test tubes and experiment with this . . . . 
|
|
|
Diachrynic
Hazard to Others
  
Posts: 226
Registered: 23-9-2017
Location: western spiral arm of the galaxy
Member Is Offline
Mood: zenosyne
|
|
Bedlasky, coordinating peroxynitrate, how cool is that! Too bad it requires inert gas, vacuum and dry ice temperatures... I would
have loved to try that. The thread you linked is also quite interesting, since hyponitrites are fascinating (always wanted to make them!), and I would
have never guessed something energetic could form from ascorbic acid, copper and nitrite 
woelen, it's a good question why no purple acid forms just from dissolving nitrite in sulfuric acid. My guess would be for now: Since
the purple acid seems to be quite unstable already by itself and even more so with oxidizing agents like O2 or worse NO2 which
according to Gmelin oxidize it quickly, my guess is that if some NO and HNO3 forms in the mixture, it is more likely that the NO leaves the
solution - it's solubility isn't that good, which is why most procedures making purple acid from NO seem to require pressure - you will have more
nitrogen in a higher oxidation state than +3 overall, and if some purple acid would form, the nitric acid will oxidize it immediately. This is all
pure speculation of course.
Good luck with the experiments!
There are many unanswered questions for me still. Does the order of addition matter, i.e. first nitrite then sulfite and reverse? What happens when I
dilute the acid before adding the salts? What happens when I separately dissolve nitrite and sulfite in sulfuric acid, then mix them? What about solid
NOHSO4 + SO2 gas, Gmelin says this should form the purple acid too? What about just adding copper sulfate to sulfuric
acid/sodium nitrite? Eventually I will try those out as well.
we apologize for the inconvenience
|
|
|
Bedlasky
International Hazard
    
Posts: 1251
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
Diachrynic: Maybe purple acid is similar complex like nitrite complex mentioned in that paper I posted.
About that energetic complex: It isn't certain, that this compound is actually copper(I) hyponitrite. There are still many unanswered questions, it
may be some sort of nitrosyl/nitrite+organic complex. Precise nature of this compound is really mystery, I myself try to find some info about it year
ago, but I didn't find anything.
Sulfuric acid + NaNO2 + metallic copper maybe work - copper would be a reducing agent.
|
|
|
Diachrynic
Hazard to Others
  
Posts: 226
Registered: 23-9-2017
Location: western spiral arm of the galaxy
Member Is Offline
Mood: zenosyne
|
|
Yeah, purple acid could be such a complex, but I imagine since N2O2+ has a free electron it could be colored just
like that. Radicals tend to have colors, see NO2 or Frémy's salt (which coincidentally is violet/purple in solution). This compound isn't that tho, even tho it is also made from nitrite and
sulfite. Frémy's salt can be isolated and is stable in water.
It probably does, I expect that to be the reason for the color in the experiment with formic acid (I assume the formic acid didn't do anything apart
from diluting). I don't expect just copper sulfate to work without a reducing agent, frankly, but it would be very surprising if it did.
we apologize for the inconvenience
|
|
|
woelen
Super Administrator
        
Posts: 8080
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I did some experimenting, and I succeeded in making some of the purple acid! It's a somewhat elusive compound, making it requires some trial and
error. The concentration of water, needed in the acid must be within fairly tight bounds. Too little or too much water, and it does not work.
For a start, you can do the following:
Take a SMALL pinch of NaNO2. A piece of 2x2x2 mm is enough. Too much nitrite and it does not work!
Do the NaNO2 in a dry test tube.
Add appr. 2 ml of conc. H2SO4.
Swirl around, until the NaNO2 has dissolved. If the acid is really concentrated, then there hardly will be any production of gas and no brown color
can be observed above the liquid.
Add a similar amount (at most 10 mm3 of solid) of solid K2S2O5 to the liquid. Na2S2O5 also can be used.
Swirl till the solid has dissolved. Some of it will be sticking on the glass. That's not a problem.
Carefully, while keeping the test tube tilted, let a single big drop of water run along the glass, through some K2S2O5, sticking to the glass until it
hits the acid.
If this is done carefully, then 2 or 3 mm of water will be floating on top of the acid, with a clearly visible purple layer between the colorless acid
and the colorless aqueous layer.
Carefully swirl the test tube so that the water mixes with more acid. If you do this, then you clearly see a purple color, and production of some
colorless gas. The liquid becomes warm, but not overly hot. This is good. If it remains cold, then the reaction does not occur (or is very slow).
If you want the purple color to last somewhat longer, then you should cool the test tube under some running tap water.
Carefully swirling more can make all liquid purple, but it might be that the color fades again and that an additional small drop of water must be
added.
It is very easy to add too much water. Just one drop too much and the color disappears and you just get bubbles of NO and a brown color above the
liquid.
This weekend I'll do some more extensive tests. For now, I have very limited time, but I could not resist taking out the test tubes. I just wanted to
see this with my own eyes. I'll try to find a procedure, which is more reliable and gives more certain results.
But right now, I am quite convinced that the purple color is not due to trace amounts of copper.
|
|
|
EthidiumBromide
Harmless

Posts: 42
Registered: 27-9-2020
Member Is Offline
Mood: Effervescent
|
|
I have also tried this now and can confirm that this color has nothing to do with the presence of copper or any other transition metal contamination.
I loosely followed the metabisulfite method described in the OP.
In about 10 ml of 96% H2SO4 i dissolved 0.5 g of NaNO2 (heating the mixture slightly speeds this up). Then I added 0.3 g of K2S2O5, again waiting for
it to dissolve (very little SO2 formed in the process). I then waited it to cool down a bit. Once it did, I started adding water dropwise with
constant stirring until an intense color developed.
Here was my result:


It surprises me how the color was a very intense purple-indigo. Again, not a result of metal contamination, as I used high purity reagents.
[Edited on 8-1-2021 by EthidiumBromide]
|
|
|
Bedlasky
International Hazard
    
Posts: 1251
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
I tried this experiment today. And I am unable to do this compound. I added small amount of NaNO2 in to the dry test tube and "dissolve it" (actually
it didn't dissolve completely and I used very small amount of NaNO2) in cca 2ml of 96% sulfuric acid and added small amount of Na2S2O5 (which also
refuse to dissolve completely). Then I added drop of distilled water. Nothing. I added second drop. Nothing. Just a gas evolution. When I added drop
of copper sulfate solution, it turn purple but for a very short time. Nickel and cobalt didn't have influence on this.
|
|
|
EthidiumBromide
Harmless

Posts: 42
Registered: 27-9-2020
Member Is Offline
Mood: Effervescent
|
|
Bedlasky, how much water did you add? I just kept slowly adding drops of water and mixing until the color started showing up.
Also, have you tried heating up the H2SO4 after adding it to the test tube? It helps in dissolving NaNO2. Maybe try using more NaNO2 as well.
|
|
|
woelen
Super Administrator
        
Posts: 8080
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
The acid can be obtained in quite high concentrations, such that even 2 cm of liquid is nearly black. I made some pictures of the compound and will
write a webpage about this. The pictures I can share here already:
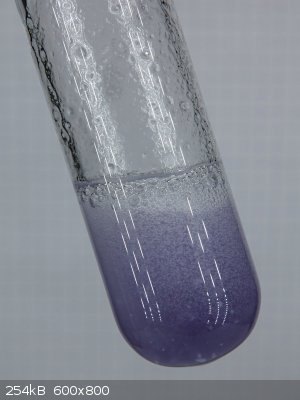  
For these experiments I used fairly high concentrations of NaNO2, but also quite high concentrations of K2S2O5. You can see solid material sticking to
the glass, not all of the solid material could be dissolved. The left picture shows high concentration, with only a little amount of water added. The
middle picture shows high concentration, with a little more water added. The right picture shows the same test tube, with bright LED-light from
behind, showing the color somewhat better than the middle picture.
I really is remarkable to see formation of this compound from only NaNO2, K2S2O5 and H2SO4.
When the dark material is diluted with water, then a nice blue liquid is obtained, like a dilute solution of CuSO4, but slightly more cyan. This most
likely is N2O3, dissolved in water. More on that follows in the webpage.
|
|
|
Bedlasky
International Hazard
    
Posts: 1251
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
So I tried this reaction again. I folowed EthidiumBromide instructions, instead of K2S2O5 I used anhydrous Na2SO3. And I obtained this beautiful
solution. I tried to add some copper nitrate which I made from piece of copper wire and nitric acid (I didn't have copper sulfate on hand), but this
caused decolorization of solution due to presence of nitrate (see the third picture).
I tried to add copper wire in to purple solution. Some purple colour appeared around copper but after a while I didn't saw further formation of this
colour.
I tried to use copper as a reductor instead of sulfite. At the surface of wire a saw some purple colour, but solution remained yellowish. When I added
drop of water, purple colour disappeared. It seems that formation of small amount of NO2 caused oxidation of this acid. This compound is really
sensitive to any oxidizing agent.
  
Btw: In this document is mentioned that N2O2+ is an intermediate step in reduction of nitrosyl in to NO. It is a radical cation.
Attachment: Trace Addition of Nitric Oxide and Nitrogen Dioxide to Air by Electrolysis.pdf (930kB)
This file has been downloaded 385 times
[Edited on 10-1-2021 by Bedlasky]
|
|
|
Diachrynic
Hazard to Others
  
Posts: 226
Registered: 23-9-2017
Location: western spiral arm of the galaxy
Member Is Offline
Mood: zenosyne
|
|
woelen, EthidiumBromide, Bedlasky, the pictures look amazing! Awesome job! It seems you obtained a
far more concentrated solution than I did, judging by how deep the color is.
The experiment that Bedlasky did is fascinating, since copper sulfate seemed to deepen the color for me, while copper nitrate caused decomposition.
Seeing how there are multiple mentions of how oxygen and nitrogen dioxide decompose the purple acid by oxidation, I'm assuming that this happend here
too, with nitric acid being the oxidizing agent. It would fit with the literature.
I'm still making slow progress on the translation of the Gmelin pages, once I am done I'll append them here, to make it accessable to non-german
speakers as well. It should be close to comprehensive for all literature on the compound up until the 1960s.
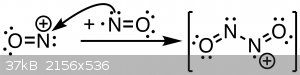
While I have not found an exact structure, I am assuming from all the facts so far the formation and structure will look something like this:
we apologize for the inconvenience
|
|
|
Bedlasky
International Hazard
    
Posts: 1251
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
Diachrynic: Actually, bonding between N and O in NO is little bit more complicated than you proposed:
https://en.wikipedia.org/wiki/Covalent_bond#One-_and_three-e...
|
|
|
Diachrynic
Hazard to Others
  
Posts: 226
Registered: 23-9-2017
Location: western spiral arm of the galaxy
Member Is Offline
Mood: zenosyne
|
|
I have never heard of that before, thank you for pointing it out. We were taught the normal bonding with a single radical... Every concept you get
taught later turns out to be horribly simplified!
we apologize for the inconvenience
|
|
|
| Pages:
1
2 |
|