Bezaleel
Hazard to Others
  
Posts: 444
Registered: 28-2-2009
Member Is Offline
Mood: transitional
|
|
Reaction between ethylenediamine and nitrite?
To a solution of NiSO4 and H2SO4, I added ethytlenediamine (en) in a ratio of 1:3 for NiSO4 and 1:1 for the H2SO4 present. I filtered out the
precipitate and added NaNO2 to the solution so that Ni2+ : NaNO2 became 1:12, with respect to the amount of NiSO4 before filtration.
In numbers, the final solution was made with:
2.77g NiSO4.6H2O
0.69g H2SO4
2.32g (en)
--> now precipitate was filtered off
8.9g NaNO2 (slightly more than 12 times the molar amount of NiSO4.6H2O)
I hoped to obtain the red Ni(en)3(NO2)2, and indeed the solution lost most of its purple colour when the (en) was added. After some standing, the
colour became more reddish, somewhat like ruby.
So far so good, but when I started to evaporate the solution, an ethanolic but sweetish smell developed. This smell has become stronger over the past
days (it's evaporating only slowly).
My question is: where could this smell come from?
|
|
|
njl
National Hazard
   
Posts: 609
Registered: 26-11-2019
Location: under the sycamore tree
Member Is Offline
Mood: ambivalent
|
|
Can you provide a reference for this procedure?
|
|
|
Bezaleel
Hazard to Others
  
Posts: 444
Registered: 28-2-2009
Member Is Offline
Mood: transitional
|
|
Not really. It is succinctly described in Russian Journal of Inorganic Chemistry, vol 9, 1964, pp 711-712.
I got the reference from Gmelins, and the article mentions that Ni with 3 moles of (en) and between 2 and 12 moles of (NO2)2- always yields
[Ni(en)3)](NO2)2.
Here are some pictures of the liquid and the crystals which now start to form, and which look like mainly NaNO2.
 
But my question is not about the complex, but about the peculiar sweetish ethanolic smell which is evolving. What could it be?
|
|
|
MidLifeChemist
Hazard to Others
  
Posts: 192
Registered: 4-7-2019
Location: West Coast USA
Member Is Offline
Mood: precipitatory
|
|
I hope it is the sweet smell of success!
|
|
|
njl
National Hazard
   
Posts: 609
Registered: 26-11-2019
Location: under the sycamore tree
Member Is Offline
Mood: ambivalent
|
|
Was there any bubbling during the reaction? That could indicate the loss of N2 via diazotization, giving various sweet smelling compounds (ethylene
and ethylene glycol come to mind). Maybe it's a stretch, but it's an idea 
|
|
|
DraconicAcid
International Hazard
    
Posts: 4356
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Weird. Why would that compound be red? Ethylenediamine should have made it, and kept it, utterly purple. Unless you've got [Ni(en)2(NO2)2], but I
didn't think nitrite would be a good enough ligand to push off an en.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Bezaleel
Hazard to Others
  
Posts: 444
Registered: 28-2-2009
Member Is Offline
Mood: transitional
|
|
Quote: Originally posted by njl  | Was there any bubbling during the reaction? That could indicate the loss of N2 via diazotization, giving various sweet smelling compounds (ethylene
and ethylene glycol come to mind). Maybe it's a stretch, but it's an idea 
|
Yes, there was. Slowly but surely. The reason I noticed is that there were always some bubbles on top of the
liquid. When I broke them, some time later there would be new bubbles. So, thanks for the thought!
Quote: Originally posted by DraconicAcid  | | Weird. Why would that compound be red? Ethylenediamine should have made it, and kept it, utterly purple. Unless you've got [Ni(en)2(NO2)2], but I
didn't think nitrite would be a good enough ligand to push off an en. |
Gmelin's states the compound is red.
The article I quoted earlier says "It is known that ethylenediamine has a great tendency to enter the coordination sphere of a complex to form a
stable ring, therefore there is a basis for considering that this ligand ((NO2)2-) should be found in the inner sphere". Maybe they're right?
My own fantasy imagines that maybe (NO2)2- bonds to the (en), thus indirectly influencing the Ni at the centre of the complex. And if what njl
suggests about daizotisation is true, then it means that there is quite a strong interaction between (en) and (NO2)2-.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4356
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Aha! https://spiral.imperial.ac.uk/bitstream/10044/1/17336/2/Hitc...
According to this, the red compound should be [Ni(en)2(NO2)2], with the nitrites being N-bonded. With bulkier diamines, the nitrites are O-bonded,
and the complex is blue.
There is also a funky [Ni(en)2(NO2)]BF4 complex with bridging nitrites, which is also red.
[Edited on 9-12-2020 by DraconicAcid]
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
woelen
Super Administrator
        
Posts: 8027
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Interesting stuff. I have (dien) as well, which is bulkier than (en) and tridentate. Maybe that gives a very different color. The Ni-(dien) complex
has the same color as the Ni-(en) complex, but with the added nitrite things may be different.
A nice weekend project . . .
|
|
|
woelen
Super Administrator
        
Posts: 8027
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I prepared solution of Ni(ClO4)2 in water. There may be slight excess of HClO4, but now much. This solution has the well-known green color of aqueous
Ni(2+).
To this, I added a large excess amount of NaNO2. This solid quickly dissolves, there was slight bubbling (probably due to the slight excess of HClO4,
which leads to formation of some N2O3 with the nitrite). The liquid then became much darker green. On boiling the liquid remains this darker green.
This must be a complex of nickel(II) with the nitrite ion.
Next, I added some (en). This leads to formation of the well-known purple solution and formation of solid purple crystals. The liquid is purple, on
heating it even becomes dark purple-blue. Next, I allowed the liquid to stand for a while to see what kind of solid material crystallizes from the
solution. The liquid now is cooling down and in a few hours I hope to get some crystals. I, however, did not see any ruby-red color, just the familiar
Ni(en)3(2+) color. Maybe perchlorate is not the best counter-ion, because it leads to quick crystallization of the Ni-(en) complex, which then is
removed from solution and does not react anymore with the nitrite.
I also have KNO2. I can use that in a similar reaction and get all perchlorate precipitated as KClO4, leaving excess NO2(-) in solution, together with
Ni(2+) and (en). Let's first see what the current solution will do.
|
|
|
Bezaleel
Hazard to Others
  
Posts: 444
Registered: 28-2-2009
Member Is Offline
Mood: transitional
|
|
That's interesting, woelen. As the pictures I posted above show, the red compound is very soluble, apparently even better than NaNO2, so I guess that,
as you say, the ClO4- is not a good counter ion in this case, because it takes the nickel out of solution.
In the very last stage of the crystals drying up, you can see the red compound group together. (The dish was shaken between photos.)

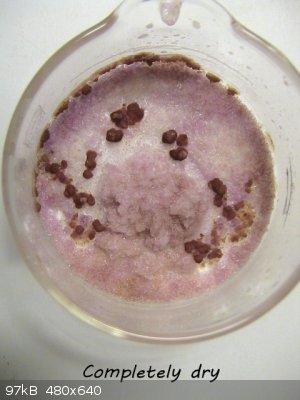
|
|
|
woelen
Super Administrator
        
Posts: 8027
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I have done some more experimenting with the nitrite/(en)/Ni system. I found that the colors strongly depend on the concentration of the nitrite ion.
- I dissolved NiSO4.6H2O in water. No perchlorate, just to be sure that the nickel remains in solution.
- To this, I added some NaNO2 (2 to 3 times the molar amount of nickel). This leads to formation of a deep emerald green solution. The color actually
is very beautiful. On internet, this complex also is described. Nickel(II) forms a somewhat unstable emeral green complex with nitrite, which slowly
dispropotionates. The nitrite disproportionates to nitrate and NO-gas. I indeed observed faint bubbling of the solution.
- Next, I added a small amount of 50% solution of (en). When this is done, I obtained a purple layer on top of the emerald green layer. On shaking, it
was mixed and the solution became very dark blue/green. I think that this is a mix of the emerald green nitrito complex and the dark blue (bis)en
complex of nickel(II).
- I split the solution in two parts, called part A and part B
- To part A, I added a few more drops of 50% (en). On shaking, this solution becomes purple, the well-known color of [Ni(en)3](2+).
- To part B, I added a lot of additional solid NaNO2. On dissolving of the NaNO2, the liquid turns red, like shown in the pictures in this thread. So,
a lot of excess nitrite ion is needed to get the red complex.
I now have the mix standing in the cold, very slowly evaporating water. But I do not have much hope for nice crystals. The liquid contains so much
NaNO2! Hard to get pure crystals. But it is interesting to see the formation of the red color and to see the conditions in which it is formed.
|
|
|
woelen
Super Administrator
        
Posts: 8027
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Another interesting observation.
I also did the experiment with KNO2, solution of Ni(ClO4)2 and ethylene diamine. Actually, this was the first experiment I did after your post. I
added potassium nitrite to the solution of Ni(ClO4)2. This immediately led to formation of a pale green precipitate and an emerald liquid was
obtained. This was a mistake, I did not take into account that K(+) and ClO4(-) form a precipitate of KClO4. Hence my further experiments were with
NaNO2.
Anyway, I had mixed the KNO2 and Ni(ClO4)2 already, and had a lot of precipitate. I decided to add ethylene diamine anyway, boiling the liquid in
order to dissolve all KClO4 again. The liquid I obtained was green/blue. From this liquid, on cooling down, again a precipitate was formed. It was
pale green. This was the impure KClO4.
I never throw away impure perchlorate waste from experiments. It is nice stuff for demos (e.g. for demos for kids, with little flashes and that kind
of things). So, I kept the solid and allowed it to dry. I expected to get a pale green powder. But, after two days, when I came back, the material was
perfectly dry, and guess what, it had turned pink! So, I am quite sure that it has some of the nitrite/(en) complex of nickel as impurity. The
material is indeed mostly KClO4 (a mix with red P and Al-powder burns very fiercely, almost explosively, when ignited)., but it has a red impurity.
|
|
|
Bezaleel
Hazard to Others
  
Posts: 444
Registered: 28-2-2009
Member Is Offline
Mood: transitional
|
|
That's interesting, woelen. The Russian article (scan added) mentions that the red complex should form always when Ni2+ : (en) : (NO2)- = 1 : 3 : 2 or
more. It also says that other red complexes exists with less (en), as also the article linked to above by DraconicAcid points out.
Gmelins Handbuch states that the red [Ni(en)3](NO2)2 forms red plates. I'll prepare some NiSO4 (ran out of it) and test whether the red plates will
form when I just use the ratio of 1 : 3 : 2 or 1 : 3 : 2.5. The use of NiSO4 as a starting point has the advantage that Na2SO4 should crystallise out
first, because its solubility is limited compared to NaNO2 and the red complexes. Using Ni(ClO4)2 and KNO2 has the same advantage (to an even larger
extent).
I'm afraid this will need to wait until coming weekend.
Attachment: Russ J Inorg Chem 1964 pp711-712 (Reaction of Nickel Acetate with Ethylenediamine and Potassium Nitrite).pdf (333kB)
This file has been downloaded 263 times
[Edited on 16-12-2020 by Bezaleel]
|
|
|
DraconicAcid
International Hazard
    
Posts: 4356
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
I honestly don't believe Gmelin in this case- [Ni(en)3]2+ is purple, and will stay purple regardless of the counterion. If it's red, then the nitrite
must be coordinated.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
woelen
Super Administrator
        
Posts: 8027
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I also think that the red compound is not an [Ni(en)3](2+) complex. It must have nitrite coordinated to nickel, and hence I expect the complex to have
at least one less (en) ligand. It could be the neutral complex Ni(en)2(NO2)2, assuming that the nitrite is coordinated through a single atom.
|
|
|
woelen
Super Administrator
        
Posts: 8027
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I indeed can try to make a solution without high concentrations of perchlorate and potassium ions, by mixing Ni(ClO4)2 and KNO2 again. This, however,
it not easy. Making a solution of Ni(ClO4)2 of precise concentration, without excess perchlorate is very hard. You always need a little excess acid to
get all basic nickel carbonate (which in itself already is a compound of somewhat variable composition) dissolved. Even if you add excess nickel
carbonate, not all acid is used up. When the concentration of acid becomes lower, then the reaction simply comes to a halt (or becomes extremely
slow). I always add extra (en) to first neutralize the excess acid, and then adding more for formation of the complex.
I think I can get the experiment such that I can have nickel(II), ethylene diammonium ions and nitrite in solution with just a little K(+) and
ClO4(-). To this I can add excess (en) for complex formation. A nice weekend experiment, which takes a little more care in its execution.
|
|
|
Boffis
International Hazard
    
Posts: 1879
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Is it possible that you are getting nitrosyl .NO complexes. There are a lot of these and their colours are often intense and contrast distinctly with
the NO free complexes. They are usually prepared either by thermal degradation of hydroxylamine or reactions with nitrite +/- a reducing agent (would
en be capable of this?).
|
|
|
woelen
Super Administrator
        
Posts: 8027
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Quote: Originally posted by woelen  | | [...]I think I can get the experiment such that I can have nickel(II), ethylene diammonium ions and nitrite in solution with just a little K(+) and
ClO4(-). To this I can add excess (en) for complex formation. A nice weekend experiment, which takes a little more care in its execution.
|
I did the above experiment last weekend. I succeeded in preparing a very concentrated solution of nickel(II) with nitrite ion. This solution has a
deep green color, with a faint brownish hue (pictures follow soon). A lot of KClO4 precipitated. I put the liquid in the freezer for a while to have
as much as possible of the perchlorate removed. A sample of this liquid was tested with a drop of concented KNO2, no further precipitate of KClO4
appeared. This solution is not entirely stable, slowly it develops bubbles of gas. Apparently, the nitrite slowly disproportionates to NO and nitrate.
After a while, however, formation of bubbles stops.
I very slowly added 50% ethylene diamine to this solution, while mixing after every two drops. In this way, I first obtained different shades of
blue/green solutions, then a greyish blue solution, then a blue/purple solution and finally a purple solution. In solution you don't get the red
complex with (en) and nitrite.
From this solution I took part and to this I added a lot more KNO2. Only then the solution turns red.
Now I have both solutions standing, the purple one and the red one, hoping to get some crystals.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4356
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Hey Woelen- you might find this helpful.
http://www.sciencemadness.org/talk/viewthread.php?tid=26095&...
I followed the first synthesis to get K4[Ni(NO2)6], but haven't gotten around to making the reported red complex.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|