yobbo II
National Hazard
   
Posts: 763
Registered: 28-3-2016
Member Is Offline
Mood: No Mood
|
|
Heating Potassium perchlorate solution
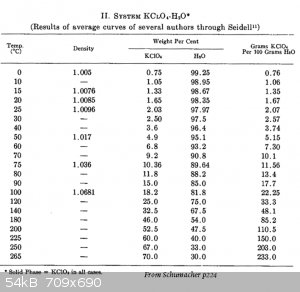
Attached is a table from The Perchlorates by Schumacher (book).
It would appear that you can heat solutions of potassium perchlorate solutions all the way up to 265C.
Is this true or am I missing something?
Yob
|
|
|
MidLifeChemist
Hazard to Others
  
Posts: 192
Registered: 4-7-2019
Location: West Coast USA
Member Is Offline
Mood: precipitatory
|
|
Well a saturated solution of KClO4 will boil at a temperature between 100 and 120 degrees. There is a formula to calculate the exact boiling point,
google boiling point elevation of electrolytes. So yeah, your missing something 
|
|
|
B(a)P
International Hazard
    
Posts: 1139
Registered: 29-9-2019
Member Is Offline
Mood: Festive
|
|
Pressure I suspect.
|
|
|
woelen
Super Administrator
        
Posts: 8012
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
At atmospheric pressure you'll get dry KClO4 a few tens of degrees above 100 C. If you continue heating to 265 C you get dry KClO4. If you go higher,
then the KClO4 decomposes, giving KCl and O2.
|
|
|
yobbo II
National Hazard
   
Posts: 763
Registered: 28-3-2016
Member Is Offline
Mood: No Mood
|
|
It's just that the pressure was not mentioned in the table or the text around the table.
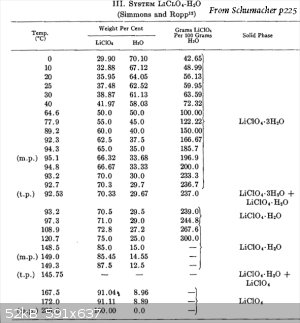
Here is another table showing Lithium perchlorate. It appears to be saying something similar, ie. you can heat the solution way up to the melting
point of Li Perchlorate.
(Li perchlorate actually has a melting point without decompositon unlike other perchlorates.)
I would be inclined to imagine that if you held a solution of Li perchlorate at 150C for a long period of time at STP, then all the water would dry
off.
t.p. is noted in some places. What is that. Also m.p. (melting point) is noted. Melting point of what? The last row I can see what m.p. means (the Li
Perchlorate).
Perhaps I need to go back to Simmons and Ropp!
Yob
|
|
|